Role of Dietary Polyphenols on Adult Neurogenesis and Cognition during Aging
© 2025 Padmanabh Singh, Nisha, Sneha Tiwari, Vijay Paramanik, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The formation of new neurons in adults is an important function that occurs only in specific brain regions. Adult neurogenesis plays an important role in maintaining normal brain homeostasis, replacing damaged neurons, neuronal plasticity, and cognitive functions. The generation of new neurons from Neuronal Stem Cells (NSCs) occurs throughout postnatal stages; however, the process is affected during aging. The decline of the Neuronal Progenitor Cell (NPC) population and the rate of differentiation of NPCs into neurons leads to decreased adult neurogenesis and cognitive functions during aging. Several factors that contribute to the age-associated decline of adult neurogenesis are changes in hormonal and neurotransmitter levels, formation of Amyloid β (Aβ) protein plaque aggregates, oxidative stress, and neuroinflammation. Due to anti-apoptotic, antioxidant, anti-inflammatory properties and other health benefits, plant polyphenols have been widely used to recover cognitive functions in animal models as well as in human subjects. Several studies show that intake of dietary polyphenols such as resveratrol, curcumin, grapeseed, blueberry extract, etc., induces adult neurogenesis and improves learning and memory in different animal models. The present article discussed the significance of dietary polyphenols and their effects on adult neurogenesis and cognition during aging.
Introduction
Neurogenesis is the process of generating functional neurons from NSCs and NPCs. Neurogenesis mostly occurs during embryonic stage (embryonic neurogenesis) and postnatal stage (adult neurogenesis). At the start of 20th century, neurogenesis was thought to exclusively occur during the embryonic stages. With the development of [H3]-thymidine cell labeling and autoradiography techniques in the 1950s and 1960s, it was discovered that neurons are also generated during the postnatal stages called adult neurogenesis [1]. Adult neurogenesis is reported both in non-mammalian and mammalian species. In fishes, adult neurogenesis is well studied in teleosts, where neurogenesis occurs in many brain regions such as dorsal and ventral telencephalon, hypothalamus, optic tectum, and cerebellum. Adult neurogenesis in teleosts is regulated by environmental factors, social interaction, rearing conditions, etc [2]. The songbird system is a well-studied adult neurogenesis system where new neurons are generated in a region called the High Vocal Center (HVC) in birds. Adult neurogenesis in birds depends on the genders and seasons [3]. In mammals, adult neurogenesis is confined to specific regions in the brain, i.e., the neocortex, Sub-Ventricular Zone (SVZ), and Sub-Granular Zone (SGZ) in the Dentate Gyrus (DG) of the hippocampus and Olfactory Bulb (OB). Using the rostral migratory route, neurons generated from the SVZ region migrated to the OB, becoming granule and periglomerular neurons. On the other hand, the neurons generated in the SGZ region undergo differentiation and integrated as granule cells in the DG [4].
Adult neurogenesis takes place throughout life. Studies in fishes, birds, and mammals showed that a decrease in the number of neuronal stem cell population that generates neurons and their microenvironment leads to a decline in adult neurogenesis during aging [5]. Several factors that reduce the neuronal stem cell population in these brain regions during aging are lowered expression of mitogens such as Epidermal Growth Factor (EGF) and Fibroblast Growth Factor (FGF) and notch signaling that promotes neurogenesis, decreased cerebral blood volume in the DG, lowering of blood vasculature due to decrease of Vascular Endothelial Growth Factor (VEGF) level, accumulation of protein aggregates due to decrease in lysosomal degradation pathways, neuroinflammation, mitochondrial dysfunction and oxidative stress [6].
Polyphenols are secondary metabolites synthesized by plants through the shikimate/polyketide pathway and present in fruits, vegetables, seeds, and nuts. Earlier studies suggested that intake of plant-derived polyphenols (i.e., genistein, curcumin, resveratrol) shows neuroprotection and improves cognition in different animal models [7]. Due to their antioxidant and anti-inflammatory properties, plant polyphenols ameliorate neuronal damage caused by age-associated mitochondrial dysfunctions, oxidative stress, and inflammation and thus prevent and improve cognition [8]. Recent studies showed that polyphenols also improve the decline of adult neurogenesis and memory during aging [9,10].
Adult Neurogenesis
The NSCs in adult brain are mostly in the quiescent stage except in the SVZ and SGZ of DG of the hippocampus. Self-renewal NSCs in the SVZ and SGZ undergo proliferation and differentiation to give rise to NPCs, neurons, and glial cells. Newly formed neurons from the SVZ and SGZ regions are integrated into the preexisting neuronal network and execute their functions. The newly generated neurons play an important role in regulating olfaction and hippocampal-dependent memory, sexual behavior, offspring recognition, etc.
The proliferative cell layer of the SVZ region contains four different types of cells. are ependymal cells, migrating neuroblast cells (type A cells), astrocytes (type B cells), and immature Transitory Amplifying Progenitor (TAP) cells (type C cells) [11]. The NSCs population of the SVZ region is Glial Fibrillar Acidic Protein (GFAP) positive cells and show properties similar to astrocytes or type B cells. After differentiation, the type B cell gives rise to GFAP-negative TAP or type C cells. These type C cells undergo differentiation to form doublecortin-positive neuroblast cells or type A cells. Using the rostral migratory system, these type A neuroblast cells move towards the OB [5]. In the OB, the type A cells differentiated into mature granule and periglomerular cells. These mature neuronal cells, after reaching the OB, show expression of glutamate and Gamma-Aminobutyric Acid (GABA) receptors, dendritic and axonal synaptic connection, and voltage-dependent electrophysiological activities [12]. The newly generated neurons make connections with the preexisting neurons of OB such as mitral and tufted cells as well as pyramidal cells of olfactory cortex. It takes around 10 days from the generation of new neurons to the incorporation of neuronal circuits to synapse formation in OB [13]. These neuronal cells are important in regulating olfaction-mediated behavioral functions [14].
Adult neurogenesis in the SGZ region of the hippocampus starts due to the proliferation of neuronal progenitor cells. It later differentiates into Dentate Granule Cells (DGCs) and glial cells [4]. It takes about 6-8 weeks from the generation of a neuron to integrate into the preexisting neuronal network to make functional synaptic connections [15]. In the first week, the newborn DGCs undergo differentiation and migrate into the granule cell layer of the DG, where DGCs start forming cellular processes [16]. In the second week, the DGCs look more like neurons. The dendritic and axonal processes extend towards the molecular layer and the CA3 region of the hippocampus. At this point, immature neurons start getting synaptic inputs from surrounding interneurons [17,18]. DGCs start forming synaptic connections in the third week with the preexisting neuronal network. At 4 to 6 weeks, DGCs show stronger synaptic connectivity with the surrounding neurons, NR2B subunit containing N-Methyl-D- Aspartate (NMDA) receptor expression, and increased long- term potentiation. Adult neurogenesis plays an important role in maintaining cellular homeostasis and the cell population of the brain as well as regulating olfactory bulb and hippocampal- dependent learning and memory.
OB-Dependent Behavior and Adult Neurogenesis
Animals use their olfactory system to detect and analyze chemical signals from surroundings through the receptors present in sensory neurons of the OB. The granule cells and periglomerular cells are glutamatergic and GABAergic and receive inputs from the mitral and tufted cells and glomeruli, [19]. Several reports showed that the rate of NSC proliferation and differentiation in the SVZ region depends on olfactory input received by the OB [14,20,21]. Further, deprivation of olfactory inputs decreased the number and length of dendrites and the spine density of granule cells in the OB [22,23]. On the other hand, anti-mitotic drugs, stress, and irradiation affect NSC proliferation, decrease neurogenesis, and impair olfaction-dependent behavior [24,25].
Adult neurogenesis in OB is regulated by different factors such as social behavior, maternal behavior, pups recognition and reproductive behavior in animal models [26]. Earlier reports showed that the adult neurogenesis is increased during the gestation period as well as during lactation and play a significant role in recognizing odors of the pups. [27,28].
Hippocampal Dependent Behavior and Adult Neurogenesis The hippocampus is an important brain region that helps to form different types of memory, i.e., spatial memory, fear memory, recognition memory, and episodic memory [29-31]. Studies in animal models show that new neurons generated and integrated in the hippocampus influence hippocampal-dependent learning and memory [15]. Adult neurogenesis is regulated by several factors, including the behavioral and cognitive states of the animals. Several behavioral paradigms influencing hippocampal- dependent cognitive function are environmental enrichment, social interaction, exposure to hippocampal-dependent spatial and recognition memory tasks, and exercise. This behavioral experience is encoded in the hippocampus as memory by altering the neuronal network of the hippocampus. Similarly, anti-mitotic drugs, stress, and irradiation also decrease neurogenesis and impair hippocampal-dependent learning and memory [32].
Hippocampal-dependent memory tasks such as spatial memory tests increase the generation and promote the survival of new neurons. Previous studies have shown that newly generated neurons are integrated into the preexisting neuronal network and regulate hippocampal-dependent memory [33]. The Morris Water Maze (MWM) test evaluates hippocampal-dependent spatial memory in rodents. MWM training increased the survival of new neurons generated seven days earlier than the water maze training. These newly generated neurons are in the excitable stage and form synapses with preexisting neuronal networks [31]. Environmental enrichment plays an important role in improving cognitive functions in animal models. Kempermann et al. reported that enriched environment increased the survival of DGCs in the hippocampus and thus promotes adult neurogenesis as compared to standard housing condition in mice [34]. The enriched environment exposed mice showed a thick granule cell layer with 15% more cells in the hippocampus as compared to control mice and associated with better spatial memory in MWM test. Apart from survival, enriched environmental conditions also help in the maturation of newly born neurons. Tashiro et al. reported that neurons that were generated within three weeks showed more activation when re-exposed to the same environmentally enriched condition and not on a new condition [35].
Adult Neurogenesis during Brain Aging
Neurogenesis occurs in specific regions in the adult brain that can form new neurons from their precursors, like NSCs. New neurons are necessary for learning and memory and maintain the brain’s structural integrity and regeneration [36]. NSCs microenvironment is known as niche of stem cells viz all cells present in niches of stem cells affect the process of neurogenesis. This microenvironment is necessary for the normal functioning of stem cells and the coordination of their behavior and interaction with organisms’ environment [36]. The neurogenic niche comprises precursor cells and their progeny, such as glia and endothelial cells, which play an important role in the process of neurogenesis such as cell-cell contacts, involving gap junctions, paracrine effects of neurotransmitters, neurotrophic factors, and growth factors as well as synaptic contacts’ control [37]. Moreover, NSC niches are present in SVZ of the lateral ventricles and SGZ of DG in the hippocampus of the adult mammalian brain [38]. In the SVZ niche, the neurogenic ability of stem cells is retained throughout adult life, but in humans, after 2 years of birth, it is greatly reduced [39]. In the adult brain, the NSC pool is comprised of quiescent and has distinct roles with activated populations.
Aging remains one of the most common modifiers of neurogenesis in the adult brain. It is associated with the reduction of the mechanisms involved in the maintenance of organisms and organ homeostasis. The aging brain has been characterized by cognitive impairments, which lead to a high risk of pathologies. Further, it negatively affects neurogenesis by decreasing cell production in both neurogenic niches of the hippocampal brain and SVZ [40]. Several studies have reported aging as a risk factor that declines the ability of the brain to produce new neurons throughout the entire life and affects hippocampal NSC activity, reducing the number of progenitors that affect the fate of newly formed cells [41].
The brain contains several distinctive features and age-related mechanisms leading to decreased neurogenesis. Moreover, aging causes structural changes in DG and SVZ neurogenic niches [42]. Moreover the SVZ gets thinner during aging, and several other significant changes have occurred ependymal and astrocytic cell morphology, and RMS tends to disappear [43]. These changes may be responsible for progressive loss of new neurons and correlate with the impairment of olfactory discrimination abilities [44]. In the hippocampus, a dependent significant increase in the quiescent to active NSCs ratio suggests a loss of NSC activity. Further, dendritic spine densities of new neurons in young and old animals were similar, whereas neurogenesis was low in the hippocampus of old mice [45]. During the formation of DGCs, synapses have been formed by the middle and inner molecular layers of the hippocampal DG; however, during aging, the density of these synapses has decreased [45,46].
Neurogenesis in OB has been involved in olfactory memory, maintenance of the structure of OB, and behavioral responses to pheromones. During aging, loss of function has been shown in OB due to a reduction in the capacity of cells to proliferate as well as a decrease in the number of Olfactory Receptor Neurons (ORNs) in the niche, which causes decreased expression of extracellular matrix genes, which involve in insulin growth factor and EGF signaling and leads to olfactory impairments [40,47]. During aging, several changes have occurred in the microenvironment of the neurogenic niche. These extrinsic and intrinsic molecular signalling regulate neurogenesis. Growth factor is important for cellular proliferation and neuronal differentiation, and useful for cellular plasticity during aging. Epidermal Growth Factor Receptor (EGFR) plays an important role in proliferation of SVZ NSPCs by activating Extracellular Signal Regulated Kinase (ERK) transduction pathways. Reports showed that both EGFR and ERK/p-ERK signaling pathways are decreased in the SVZ region during aging [44,48]. Further, defects in the NSCs lysosomes activity led to the increased formation of protein aggregates that affect the proliferation and activation of NSCs decreased with age [36].
Physical activity and an enriched environment might potentially regulate hippocampal adult neurogenesis and OB neurogenesis. Exercise maintains the level of cognitive functions and loss of physical activity due to aging [49]. During aging, NSCs lose their ability to proliferate and become quiescent; however, they can be reactivated by certain stimulations like exercise. Recently, it has been demonstrated that High Mobility Group B2 (HMGB) family protein is important for the transition of NSCs from quiescence to proliferation. Aging negatively regulates these cell populations while running exercise and stimulates the proliferation of HMGB2+ cells. It can be a novel marker for identifying NSC activation in the adult hippocampus [50]. The regulation of hormones is also an important aspect of aging. Estrogen has neuroprotective properties. Its deficiency during menopause may cause cognitive impairments. In the adult female, high levels of estrogen lead to increased hippocampal cell proliferation [51].
Acute stress may reduce NSC proliferation in the adult brain. Corticosteroids that are important for maintaining the homeostasis of the Hypothalamic-Pituitary-Adrenal Axis (HPA-axis). HPA-axis dysregulation involves several psychiatric disorders with high levels of Glucocorticoids (GCs). Corticosteroids are involved in declining of adult hippocampal neurogenesis with age. It has been demonstrated that adrenalectomy restored neurogenesis in adults like younger animals [37]. Chronically increased GC levels might be one of the reasons behind the reduced neurogenesis during aging and age-related memory decline. Mifepristone, a GC receptor antagonist, up-regulated the neurogenesis in the hippocampus and improved cognitive function in old rats. Similarly, inhibition of GC-mediated singling increased adult neurogenesis in aging rats. During aging, microglia get activated in the SVZ niche and secrete proinflammatory cytokines, resulting in an unfriendly environment for NSCs and, subsequently, reduce neurogenesis.
Oxidative Stress and Adult Neurogenesis during Aging Reactive Oxygen Species (ROS) are generated from normal cellular processes. These important metabolic products have beneficial functions and deleterious effects on cells or tissues. In the cell, oxygen free radicals are generated in the electron transport chain during oxidative phosphorylation. These oxygen free radicals include singlet oxygen (1O2), superoxide (O2•–), hydroxyl free radicals (HO•), peroxides (O22–), etc. Apart from normal cellular processes, ROS are also generated due to irradiation, pollution, metal toxicity, smoking, radiation, etc [52]. Aging is one of the important factors associated with an imbalance in the production and elimination of free radicals, which results in accumulation of ROS. Age-associated increase in ROS levels leads to damage of cellular and mitochondrial membrane damage, protein oxidation, and DNA damage that affects cellular homeostasis, neurodegeneration, and cognitive dysfunction [53].
Mitochondrial dysfunction and the accumulation of oxidative free radicals are important cellular and biochemical factors that affect the NSC population during aging. Due to high energy demand, NSCs accumulate high oxidative free radicals. Previous studies showed that oxidative stress has positive and deleterious effects on the NSC population. During normal physiological conditions, oxidative free radicals play an important role in regulating NSC proliferation, differentiation, synapse formation, and neurotransmission. In vitro and in vivo studies showed that activation of NADPH oxidase is important for ROS generation and NSCs proliferation through protein kinase B (PI3K)/Akt signaling pathway. Further, with an increase in ROS level, the proliferation of NSCs decreased, and differentiation increased [54-56]. On the other hand, high oxidative free radicals generated due to an imbalance of its production and elimination show harmful effects and lead to neuronal cell death.
Antioxidant enzyme systems are crucial for scavenging oxidative free radicals and protecting cells from oxidative damage. SOD is one of the antioxidant enzymes that protect cells from oxidative stress by breaking down superoxide ions into O2 and H2O2. Earlier reports showed that the activity of SOD decreased in the brain during aging [57,58]. Analysis of genetic knockout models showed that SOD plays an important role in the proliferation and differentiation of NSCs during adult neurogenesis. CuZnSOD and MnSOD heterozygous knockout mice showed impaired adult neurogenesis with reduced BrdU+/NeuN+ positive cells in the SGZ region of the hippocampus [59]. Similarly, Rola et al. reported that EC-SOD mutant mice showed a 40% reduction in the BrdU+/NeuN+ positive neuronal cells in the hippocampus [60]. Therapeutic irradiation is one of the major factors affecting adult neurogenesis through ROS generation. Due to reduced antioxidant enzyme activities, aging is more susceptible to radiation-mediated oxidative stress and cognitive dysfunction. Casciati et al. reported that radiation damaged hippocampal mitochondria, induced apoptosis, and decreased NSCs population and adult neurogenesis (NeuN+ positive cells) and effects of radiation increased with aging [61]. Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that induces expression of genes involved in the scavenging of ROS and cytoprotection. NRF2 is also plays an important role in adult neurogenesis and associated learning and memory function. However, the expression of NRF2 at both mRNA protein levels decreased in the brain during aging [62,63]. Robledinos-Antón et al. analyzed the role of NRF2 in a genetic mutant mouse model. They reported that NRF2 knockout mice showed impaired NSC proliferation, altered neuronal to glial cell differentiation in the SGZ region of the hippocampus, and cognitive dysfunction. This shows a decrease in the activities of the antioxidant enzyme system and proteins involved in various pathways that affect adult neurogenesis and cognitive function during aging [64].
Inflammation and Adult Neurogenesis During Aging Inflammation is a complex cellular and molecular response that attempts to respond to injury, clear pathogens, and destroy or damage host cells. Cellular and molecular changes due to immune response in the neurogenic niche affect adult neurogenesis [40]. In the brain, microglia, astrocytes, and circulatory macrophages play an important role in maintaining the production of inflammatory cytokines in the form of either pro-inflammatory cytokines or anti-inflammatory cytokines and chemokines. These mediators may alter the NSC’s niche. During inflammation, microglial cells activate and produce proinflammatory cytokines and chemokines such as Interleukins (ILs), Tumor Necrosis Factor-Α (TNF- α), Transforming Growth Factor-β (TGF- β), interferon-ϒ (INF- ϒ) [65].
Proinflammatory cytokines affect neurogenesis by several pathways. IL-6 proinflammatory cytokines decrease neurogenesis and responsible for the initiation of the JAK/ STAT3 pathway, which activates astrocyte differentiation [66]. Further, RAF/MEK/ ERK pathway modulates the JAK/STAT3 pathway by regulating gp130 expression in NSCs henceforth, switching the process of NSCs neurogenesis to astrocytogenesis. Jia et al. suggested that gp130 is a therapeutic target for increasing neurogenesis [67]. Briefly, treated with recombinant TNF- α on cultured NSCs causes decreased expression of a neuron-specific cytoskeletal protein, microtubule-associated protein (MAP)-2, suggesting that TNF-α might have inhibitory effects on neuronal survival and differentiation. Further, in vitro and in vivo studies have shown that the administration of IL-1β decreases the rate of hippocampal NSC proliferation, attributed to the nuclear factor-kappa B (NF- κB) pathway [68]. During aging Sub Ependymal Zone (SEZ) is mediated with proinflammatory cytokines [69,70].
Chemokines are secreted signaling proteins that can guide migrating cells by increasing the concentration of chemokine receptors in attracting cells. Chemokines in the Central Nervous System (CNS) and their associated receptors are widely expressed in NSCs, such as Stromal Cell-Derived Factor-1 alpha (SDF- 1α)/ C-X-C chemokine receptor type 4 (CXCR4). Moreover, activated SDF- 1α gave single to NSCs for migration from neuronal damage site and enhanced NSCs proliferation by activating Akt1 and Forkhead box O (FOXO3a) singling [71,72]. FOXO3a is a transcription factor that leads to the downstream target of Akt-1, and is important for regulating NPCs proliferation and cell cycle control. Several studies have shown that microglia have dual roles according to their situation, regulating adult neurogenesis by either activating or inhibiting in both intact or injured brains. Microglial activation and inflammation can be dangerous for adult neurogenesis despite some other studies have shown that microglial is beneficial for neurogenesis under certain conditions [73,74].
Adult neurogenesis is also regulated by immune cells. CD4 T cells promote adult neurogenesis by regulating insulin growth factor 1 (IGF-1), which is involved in the production of BDNF in the DG of the hippocampus. IGF-1 helps in NSC proliferation and increases the number of granule cells in DG [75]. Th4 cells are mostly detrimental via the release and action of cytokine IFN-γ. On the other hand, Th2 cells are neuroprotective via the release and action of its main anti-inflammatory cytokine, IL-4. IL-4-stimulated microglia in the presence of IGF-1 and IFN-γ and induced neurogenesis [76]. TGF-β involved in cell growth, differentiation, migration, and apoptosis. TGF-β ligands bind to the TGF-β receptor kinase and activate Smads (R-Smads). In mammals, TGF- β has three isomers β1, β2, and β3. Studies have shown that in Smad3 null mice, these play an important role in neurogenesis and colocalization with mature neuron marker neuronal nuclei (NeuN) in the DG of the hippocampus. Moreover, deficiency of Smad3 showed disrupted neuronal proliferation and migration and decreased DG neurons compared to wild-type mice [77,78]. Further, CD8 T cells release granzyme B (GrB), which inhibits neurogenesis via activation of the Giα/Go-coupled receptor. Stimulation in these receptors causes a decreased level of cyclic AMP, which increases Kv1.3 channels on NSCs [77]. Kv1.3 is also responsible for neurotoxicity. Studies have suggested that blocking of Kv1.3 channels increased the NSCs level of differentiation [77,79,80]. In aged mice T cell infiltration in neurogenic niches decreased the NSCs proliferation [70].
Inflammation also has been caused by bacterial components, e.g., lipopolysaccharide (LPS). LPS is a constituent of gram-negative bacterial cell walls and binds with toll-like receptor 4 (TLR-4), mainly expressed in the brain by microglia, astrocytes, and neural precursor cells. TLRs activate NF-κB, a transcription factor that induces the production of main proinflammatory mediators IL-1β, IL-6, and TNF-α [81]. Moreover, LPS decreased neural precursor cell proliferation through activation of TLR-4. Several studies have shown that LPS negatively regulates neurogenesis by affecting type 2 progenitor cells, decreasing the number of newborn cells in DG, and is responsible for hippocampal memory impairment [82-84]. Seong et al. suggested that injection of LPS to 7-week- old mice activates TLR4-NF-κB signaling in the hippocampus and induces proinflammatory cytokines. Thereafter, treated with Epigallocatechin-3-Gallate (EGCG), a polyphenolic flavonoid found in green tea, it restores the proliferation of NSCs in DG and ameliorates NSCs apoptosis [85].
Histamine, a biogenic amine associated with allergic and inflammatory reactions, is also involved in inflammation and regulates adult neurogenesis. Histamine receptor 1 (HR1) and (HR4) promote the production of proinflammatory cytokines TNF-α and IL-6. HR1, HR2, and HR3 express in NSCs niche and spatially induced NSCs differentiation and proliferation through the HR2 and HR3 singling pathways. Histamine induces some SVZ neuroblasts, which can migrate towards the OB and differentiate into mature neurons [86]. Proinflammatory cytokines also indirectly regulate neurogenesis via the HPA axis. Stimulation in the HPA-axis rises to a high level of GCs. Studies have shown that GCs inhibit cell proliferation and neurogenesis in DG. It also stimulates glutamate from the hippocampus, which decreases cell proliferation in DG [49].
Antioxidative and Anti-Inflammatory Effects of Plant Polyphenols and Cognition
The importance of diet in healthful aging is well acknowledged, leading to increased studies into the scientifically supported functional qualities of common foods. Polyphenols are bioactive secondary plant metabolites naturally produced and classified under nutraceuticals based on their chemical structure [8]. Fruits, vegetables, grains, and other dietary sources, such as spices, dry fruits, legumes, and herbs, contain polyphenols. Polyphenols are classified into the following groups based on the number of phenol rings and attached elements: stilbenes (e.g., resveratrol) present mainly in red fruit, grapes, peanuts, and wine, among others; flavonoids (e.g., quercetin, catechins, and kaempferol) present in red wine, onions, curly kale, leeks, broccoli, and blueberries; phenolic acids (e.g., Benzoic acids, cinnamic acid) abundant in red fruit such as strawberries, raspberries, and blackberries. More precisely, polyphenols can be found up to 200-300mg per 100g fresh weight in fruits like apples, grapes, pears, cherries, and other berries [87]. Consuming diets rich in polyphenols reduces the risk of cognitive impairments, a slower rate of cognitive decline, and better local memory performance [88,89].
Several studies showed that dietary polyphenols are neuroprotective due to their antioxidant and anti-inflammatory properties. These properties protect brain cells by reducing the oxidative and inflammatory-mediated damage to neuronal cells. Dietary polyphenols possess one or more aromatic rings bonded to a hydroxyl group. This feature makes them good electron or hydrogen atom donors, neutralizing ROS [90]. Oxidative damage to cell constituents, DNA, proteins, and lipids accumulates with age and leads to the degeneration of neuronal cells [91]. At a cellular level, phenolic compounds are observed to be the largest group of natural antioxidants in the human diet and anti-inflammatory factors elevated by modulating signal transductions [92]. Presence of bioactive compounds confers major function to neutralize free radicals also preventing the deterioration of aging, decreasing oxidative injury and showing anti-inflammatory effects. Antioxidant capabilities of plant-derived bioactive substances have been researched in human subjects and animal models to improve cognitive health throughout aging and neurodegenerative illnesses. Their anti-inflammatory properties, such as radical scavenging and cellular activity modulation occur in inflammatory cells.
Polyphenols are important plant derived secondary metabolites that have the potential to increase cognitive performance including learning and memory. Research on different animal models suggest that blueberries [93,94] and strawberries are helpful at restoring age-related deficits in spatial working memory, enhancing object recognition memory, and regulating inhibitory fear conditioning. It has also been found that flavonoid-rich food and beverages improve psychomotor performance in elderly animals. In addition to berries, pure flavanols like quercetin [95] and rutin [96] as well as Ginkgo biloba [40] have been found to reverse neural and behavioral aging. The antioxidant effects of resveratrol on the cognitive function of aged rats were investigated by Navarro-et al. [97]. Chronic resveratrol administration lowered nitrite and malondialdehyde levels in brain and improved hippocampal-dependent recognition memory throughout aging. Polyphenols may modify chronic inflammatory processes during cytokine production, amplify NF- kβ mediated inflammatory gene expression, and release the anti- inflammatory cytokine, transforming growth factor-beta.
Inflammatory responses against various inflammatory stimuli (oxidative stress, cytokines, excess corticosterone) are characteristic of senescent tissues and organs. In a stress-induced brain, where activation of the inflammasome and another proinflammatory signaling cascade, such as the NF-κβ signaling pathways, leads to neuroinflammation [98]. Neuroinflammation connected with aging can occur from many causes like accumulation of cell damage, including oxidative damage, failure of responses of the immune system, the natural tendency of senescent cells to secrete proinflammatory cytokines and deregulation of the autophagy system [99-101]. Once neuroinflammation occurs, deleterious effects on memory, synaptic formation, impaired synthesis of catecholamines and serotonin, and impaired adult neurogenesis [9,102]. Further, increasing attention is being paid to novel anti- aging strategies oriented toward the attenuation of the molecular mechanisms that regulate this proinflammatory state, which seem to be related to the modulation of the NF-κB signaling pathway and the antiaging protein SIRT1 for maintaining cognitive health.
Role of Plant Polyphenols on Adult Neurogenesis during Aging Adult neurogenesis is a largely preserved phenomenon that includes variations in adult-born neurons compelling spatial and associative memory impairments. Several pieces of evidence suggest that complete diets could be an adaptable factor of adult neurogenesis and cognitive health. In this context, effect of polyphenols in reducing cognitive abnormalities in aged adults and protecting against common degenerative and chronic illness that are known to be caused by oxidative stress has attracted substantial attention [103]. Polyphenols may protect brain in a variety of ways, such as by protecting susceptible neurons and improving existing neurogenesis. With age, decline in cognitive behavior has been characterized by compromised neuronal plasticity, aberrant neurogenesis, and neural death. Polyphenols are characterized by lipophilic nature and can cross the blood brain barrier. It functions as an exogenous molecule for adult neurogenesis regulation. Hayashi et al. examined that supplementation of diet enriched in polyphenols induced neurogenesis in SVZ and hippocampus of adult mice [104].
The aged brain can be characterized by an imbalance in metabolic function, changes in brain vasculature, and a decline in adult neurogenesis, which reduces the number and function of NSCs and NPCs [105,106]. These lead to a decline in cognitive health, loss of working and episodic memory, impaired learning capacity, and motor coordination, not only in the context of human neurodegenerative disorders but also during normal aging. Polyphenols also help in the prevention of the age-dependent decrease of monoaminergic neurotransmitters (e.g., serotonin, dopamine, and noradrenaline) in old rats (20 months) after chronic treatment with the polyphenols resveratrol, silymarin, quercetin and naringenin [9,107]. This is important for synaptic plasticity, memory, and the modulation of other aging-related processes, such as neuroinflammation [108]. Polyphenols may possibly lower cognitive deterioration rates in aged rats, ameliorating working memory, learning, and motor functions [9]. Similarly, Shukitt et al. observed that old rats of 19 to 21 months showed improved motor and cognitive performance after a 2% strawberry-supplemented diet [109]. Further, these rats showed enhanced hippocampal neurogenesis. Reports also suggested that polyphenols can prevent or delay age-associated neurodegenerative changes, at least in animal models of Alzheimer’s disease [110]. Polyphenols have been pointed out as exogenous molecules that modulate adult neurogenesis [111]. Adult mice’s SVZ and hippocampus have been demonstrated to stimulate neurogenesis after 40 days of treatment with a diet rich in polyphenols and polyunsaturated fatty acids [112]. Several adult neurogenesis indicators, including several newly produced SGZ and SVZ cells, were shown to be up-regulated compared to the control diet, with considerably more cells expressing neuroblast markers. This is consistent with a polyphenolic diet that positively affects the proliferation and differentiation of neuronal populations.
Sarubbo et al. reported that rats given polyphenols had higher co- localization of cell proliferation marker 5-bromo-2′-deoxyuridine (BrDU) and NeuN in mature neurons-in the granule layer, indicating greater production of new neurons [9]. Further, low concentrations of curcumin stimulate neuronal differentiation of multipotent mouse neural progenitor cells in vitro, and increased adult neurogenesis has been documented in vivo in mice fed with polyphenols. Increase hippocampal adult neurogenesis in a dose-dependent and time-dependent manner and positively affect memory. Furthermore, Flowers et al. showed that NT-020, a natural supplement based on the combination of polyphenols from blueberry and green tea, increases the proliferation of neural progenitors and improves cognitive function in aged rats, likely through the attenuation of hippocampal inflammation and the enhancement of proneurogenic signaling pathways in the same region [113].
Polyphenols have a wide range of neuroprotective effects on the brain, including the control of immune cells and adaptive stress responses, as evidenced by multiple studies [114]. Polyphenols may also have an effect on supporting cells such as astrocytes or microglia and have a direct influence on brain progenitor cells [115]. These findings highlighted the use of polyphenols in stem cell-based neurodegenerative disease therapy. In aged rats given NT-020 for four weeks, neural progenitor proliferation and spatial memory performance can improve, with lower microglial activation [113]. In adult rats, resveratrol (20mg/kg body weight) substantially enhanced the number of newly produced cells in the hippocampus, accompanied by overexpression of phosphorylated CREB and SIRT1, proteins involved in neuronal survival [116]. According to the findings, resveratrol’s effects on neurogenesis are dose and context-dependent; however, lower concentrations have a positive impact on adult NPC proliferation and survival, as well as hippocampal neurogenesis in aged rats, indicating its potential as a compound for restorative therapies against age- related brain disorders [116].
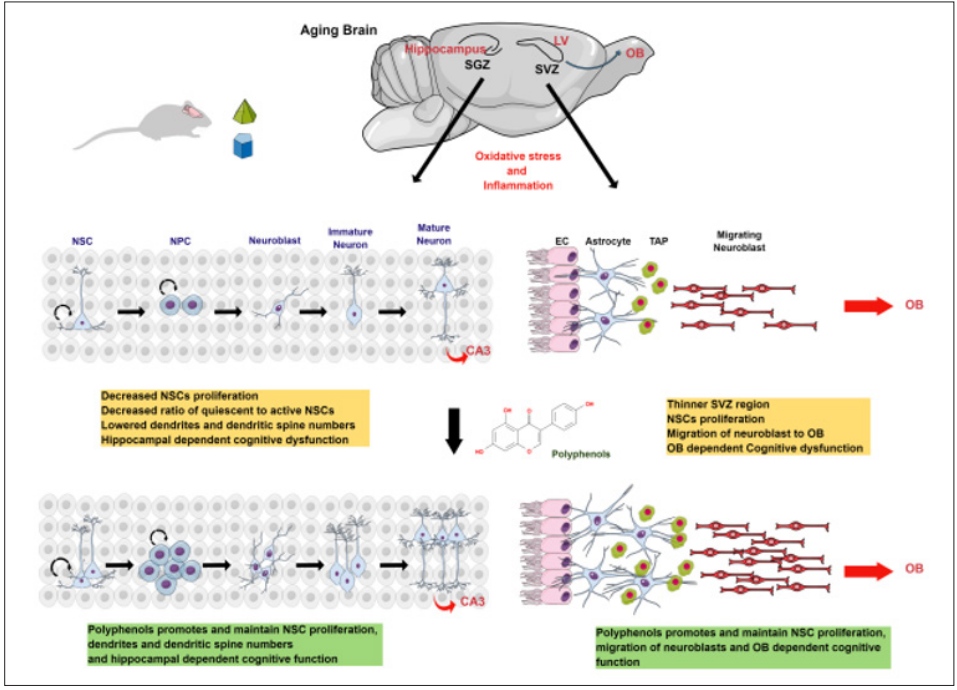
Figure 1: Schematic Diagram Representing the Role of Polyphenols on Age-Associated Changes in Adult Neurogenesis and Cognition
Oxidative stress and inflammation decreased the proliferation and differentiation of NSCs in the hippocampus and the SVZ region and their migration to OB. These age-associated changes affect hippocampal and OB-dependent cognitive functions. Further, dietary supplementation of plant polyphenols during aging promotes and maintains NSC proliferation, differentiation, and migration and thus improves cognitive functions.
Conclusion
The neurogenic niches of SVZ and SGZ regions in the brain constantly produce neurons in the postnatal stage and play an important role in maintaining brain homeostasis and cognitive function. However, biochemical and physiological changes such as oxidative stress and inflammation show harmful effects on these neurogenic niches during aging, affecting the proliferation and differentiation of NSCs and associated cognitive functions. Studies in different animal models show that dietary supplementation of polyphenols is neuroprotective due to their antioxidant and anti-inflammatory properties. Further, polyphenols activate NSCs proliferation, survival, and differentiation as well as improve cognitive and motor functions in aged animals (Figure 1). Though polyphenols show promising results in improving adult neurogenesis during aging, more research is needed to understand the details of molecular mechanisms. Understanding different molecular pathways may be beneficial to identifying new targets and designing new molecules to recover or improve adult neurogenesis during aging and age-associated neurodegenerative disorders.
Competing Interests: The author(s) declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Acknowledgments: PS acknowledges Department of Biotechnology (DBT), Government of India, for the DBT- Research Associate Fellowship. (Award Letter No. DBT- RA/2021/January/N/807). Nisha and ST acknowledge University Grants Commission (UGC) for UGC non-NET Fellowship. VP acknowledges SERB (Science and Engineering Research Board) (Registration No. SERB/LS-200/2013) Government of India, for providing financial support for the work.
Ethical Statement: Not applicable
References
- Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223-250.
- Ganz J, Brand M (2016) Adult neurogenesis in fish. Cold Spring Harb Perspect Biol 8: 019018.
- Nottebohm F (2005) The neural basis of birdsong. PLoS Biol 3: 164.
- Leal-Galicia P, Chávez-Hernández ME, Mata F, Mata- Luévanos J, Rodríguez-Serrano LM, et al. (2021) Adult neurogenesis: a story ranging from controversial new neurogenic areas and human adult neurogenesis to molecular Int J Mol Sci 22: 1148.
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H (2010) When neurogenesis encounters aging and disease. Trends Neurosci 33: 569-579.
- Lennington JB, Yang Z, Conover JC (2003) Neural stem cells and the regulation of adult neurogenesis. Reprod Biol Endocrinol 1: 9.
- Mas-Bargues C, Borrás C, Viña J (2022) The multimodal action of genistein in Alzheimer’s and other age-related diseases. Free Radic Biol Med 183: 127-137.
- Singh P, Sivanandam TM, Konar A, Thakur MK (2021) Role of nutraceuticals in cognition during aging and related disorders. Neurochem Int 143: 104928.
- Sarubbo F, Moranta D, Pani G (2018) Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and Neurosci Biobehav Rev 90: 456-470.
- Rodríguez-Vera D, Abad-García A, Vargas-Mendoza N, Pinto- Almazán R, Farfán-García ED, et al. (2022) Polyphenols as potential enhancers of stem cell therapy against neurodegeneration. Neural Regen Res 17: 2093-2101.
- Alvarez-Buylla A, Garcia-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22: 629-634.
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ (2003) Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci 23: 10411-10418.
- Whitman MC, Greer CA (2007) Synaptic integration of adult- generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci 27: 9951-9961.
- Lledo PM, Valley M (2016) Adult olfactory bulb Cold Spring Harb Perspect Biol 8: 018945.
- Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339-350.
- Zhao C, Teng EM, Summers RG, Jr Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26: 03-11.
- Espósito MS, Piatti VC, Laplagne, DA, Morgenstern NA, Ferrari CC, et al. (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic J Neurosci 25: 10074-10086.
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL (2006) Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci 26: 2326-2334.
- Shepherd GM (2004) Oxford University Press UK (5th ed).
- Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, et al. (2010) Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory FASEB J 24: 2355-2363.
- Mastrodonato A, Barbati SA, Leone L, Colussi C, Gironi K, et al. (2018) Olfactory memory is enhanced in mice exposed to extremely low-frequency electromagnetic fields via Wnt/β-catenin dependent modulation of subventricular zone neurogenesis. Sci Rep 8: 262.
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, et al. (2005) Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46: 103-116.
- Kelsch W, Lin CW, Mosley CP, Lois C (2009) A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J Neurosci 29: 11852-11858.
- Lazarini F, Mouthon MA, Gheusi G, de Chaumont F, Olivo- Marin JC, et al. (2009) Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One 4: 7017.
- Belnoue L, Grosjean N, Ladevèze E, Abrous DN, Koehl M (2013) Prenatal stress inhibits hippocampal neurogenesis but spares olfactory bulb PLoS One 8: 72972
- Feierstein CE (2012) Linking adult olfactory neurogenesis to social behavior. Front Neurosci 6: 173.
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, et al. (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by Science 299: 117-120.
- Yamaguchi M, Mori K (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA 102: 9697-9702.
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, et al. (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103: 17501-17506.
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S (2006) Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16: 296-304.
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, et al. (2007) Spatial learning depends on both the addition and removal of new hippocampal PLoS Biol 5: 214.
- Yau SY, Li A, So KF (2015) Involvement of adult hippocampal neurogenesis in learning and Neural Plast 2015: 717958.
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, et al. (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal Proc Natl Acad Sci USA 100: 14385-14390.
- Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386: 493-495.
- Tashiro A, Makino H, Gage FH (2007) Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci 27: 3252-3259.
- Isaev NK, Stelmashook EV, Genrikhs EE (2019) Neurogenesis and brain aging. Rev Neurosci 30: 573-580.
- Klempin F, Kempermann G (2007) Adult hippocampal neurogenesis and Eur. Arch. Psychiatry Clin Neurosci 257: 271-280.
- Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain significant answers and significant Neuron 70: 687-702.
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, et al. (2011) Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382-386.
- Smith LK, White CW3rd, Villeda SA (2018) The systemic environment at the interface of aging and adult Cell Tissue Res 371: 105-113.
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP (2010) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31: 151-161.
- Katsimpardi L, Lledo PM (2018) Regulation of neurogenesis in the adult and aging Curr Opin Neurobiol 53: 131-138.
- Capilla-Gonzalez V, Herranz-Pérez V, García-Verdugo JM (2015) The aged brain: genesis and fate of residual progenitor cells in the subventricular Front Cell Neurosci 9: 365.
- Lupo G, Gioia R, Nisi PS, Biagioni S, Cacci E (2019) Molecular mechanisms of neurogenic aging in the adult mouse subventricular J Exp Neurosci 13: 1179069519829040
- Morgenstern NA, Lombardi G, Schinder AF (2008) Newborn granule cells in the ageing dentate gyrus. J Physiol 586: 3751-3757.
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M (1992) Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus 2: 437-444.
- Bhatia-Dey N and Heinbockel T (2021) The olfactory system as marker of neurodegeneration in aging, neurological and neuropsychiatric Int J Environ Res Public Health 18: 697653.
- Aguirre A, Rubio ME, Gallo V (2010) Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467: 323-327.
- Fabel K, Kempermann G (2008) Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med 10: 59-66.
- Kimura A, Matsuda T, Sakai A, Murao N, Nakashima K (2018) HMGB2 expression is associated with transition from a quiescent to an activated state of adult neural stem cells. Dev Dyn 247: 229-238.
- Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19: 5792-5801.
- Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in Front Environ Sci 2: 53.
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM (2011) Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int 58: 447-457.
- Smith J, Ladi E, Mayer-Proschel M, Noble M (2000) Redox state is a central modulator of the balance between self- renewal and differentiation in a dividing glial precursor Proc Natl Acad Sci USA 97: 10032-10037.
- Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K (2010) Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int 56: 740-746.
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, et al. (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8: 59-71.
- Vanella A, Geremia E, D’Urso G, Tiriolo P, Di Silvestro I, et (1982) Superoxide dismutase activities in aging rat brain. Gerontology 28: 108-113.
- Tsay HJ, Wang P, Wang SL, Ku HH (2000) Age-associated changes of superoxide dismutase and catalase activities in the rat brain. J Biomed Sci 7: 466-474.
- Fishman K, Baure J, Zou Y, Huang TT, Andres-Mach M, et al. (2009) Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or Free Radic Biol Med 47: 1459-1467.
- Rola R, Zou Y, Huang TT, Fishman K, Baure J, et (2007) Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med 42: 1133-1132.
- Casciati A, Dobos K, Antonelli F, Benedek A, Kempf SJ, et al. (2016) Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 7: 28040-28058.
- Zhang H, Davies K, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in Free Radic Biol Med 88: 314-336.
- Matsumaru D, Motohashi H (2021) The KEAP1-NRF2 system in healthy aging and Antioxidants (Basel) 10: 1929.
- Robledinos-Antón N, Rojo AI, Ferreiro E, Núñez Á, Krause KH, et al. (2017) Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol 13: 393-401.
- Das S, Basu A (2008) Inflammation: a new candidate in modulating adult J Neurosci Res 86: 1199-1208
- Peng H, Sun L, Jia B, Lan X, Zhu B, et (2011) HIV-1- infected and immune-activated macrophages induce astrocytic differentiation of human cortical neural progenitor cells via the STAT3 pathway. PLoS One 6: 19439.
- Jia C, Keasey MP, Malone HM, Lovins C, Sante RR, et al. (2019) Vitronectin from brain pericytes promotes adult forebrain neurogenesis by stimulating CNTF. Exp Neurol 312: 20-32.
- Veerasammy S, Van Steenwinckel J, Le Charpentier T, Seo JH, Fleiss B, et (2020) Perinatal IL-1β-induced inflammation suppresses Tbr2+ intermediate progenitor cell proliferation in the developing hippocampus accompanied by long-term behavioral deficits. Brain Behav Immun Health 7: 100106.
- Belenguer G, Duart-Abadia P, Jordán-Pla A, Domingo-Muelas A, Blasco-Chamarro L, et (2021) Adult neural stem cells are alerted by systemic inflammation through TNF-α receptor signaling. Cell Stem Cell 28: 285-299.e9.
- Bitar M, Weissleder C, North HF, Clearwater MS, Zalucki O, et (2022) Identifying gene expression profiles associated with neurogenesis and inflammation in the human subependymal zone from development through aging. Sci Rep 12: 40
- Wu Y, Peng H, Cui M, Whitney NP, Huang Y, et (2009) CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem 109: 1157-1167.
- Chen Y, Yuan F, Lin J, Zhang X, Luo J, et al. (2021) Curcumin promotes the proliferation, invasion of neural stem cells and formation of neurospheres via activating SDF-1/ CXCR4 axis. Folia Neuropathol 59: 152-160.
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, et al. (2010) Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol 20: 11-17.
- Li L, Gan H, Jin H, Fang Y, Yang Y, et (2021) Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int Immunopharmacol 92: 107335.
- Araki T, Ikegaya Y, Koyama R (2021) The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur J Neurosci 54: 5880-590.
- Wang B, Jin K (2015) Current perspectives on the link between neuroinflammation and Metab Brain Dis 30: 355-365.
- Wang Y, Symes AJ (2010) Smad3 deficiency reduces neurogenesis in adult mice. J Mol Neurosci 41: 383-396.
- Hiew LF, Poon CH, You HZ, Lim LW (2021) TGF-β/Smad signalling in neurogenesis: implications for neuropsychiatric Cells 10: 1382.
- Wang T, Lee MH, Choi E, Pardo-Villamizar CA, Lee SB, et al. (2012) Granzyme B-induced neurotoxicity is mediated via activation of PAR-1 receptor and 3 channel. PLoS. One 7: 43950.
- Zhou YY, Hou GQ, He SW, Xiao Z, Xu HJ, et al. (2015) Psora-4, a Kv1.3 blocker, enhances differentiation and maturation in neural progenitor cells. CNS Neurosci Ther 21: 558-567.
- Lawrence T (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol 1: 00165.
- Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO (2014) Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci 8: 83.
- Perez-Dominguez M, Ávila-Muñoz E, Domínguez- Rivas E, Zepeda A (2019) The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regen Res 14: 817-825.
- Domínguez-Rivas E, Ávila-Muñoz E, Schwarzacher SW, Zepeda A (2021) Adult hippocampal neurogenesis in the context of lipopolysaccharide-induced neuroinflammation: A molecular, cellular and behavioral review. Brain Behav Immun 97: 286-302.
- Seong KJ, Lee HG, Kook MS, Ko HM, Jung J, et al. (2016) Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in Korean J Physiol Pharmacol 20: 41-51.
- Saraiva C, Barata-Antunes S, Santos T, Ferreiro E, Cristóvão AC, et al. (2019) Histamine modulates hippocampal inflammation and neurogenesis in adult Sci Rep 9: 8384.
- Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Wozniak K, et al. (2020) Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr 60: 626-659.
- Paramanik V, Kurrey K, Singh P, Tiwari S, Nisha (2023) Roles of genistein in learning and memory during aging and neurological disorders. Biogerontology 24: 329-346
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, et (2016) oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev 2016: 743279796.
- Poulose SM, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive Adv Nutr 15: 804-811.
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45: 287-306.
- Dragsted LO (2003) Antioxidant actions of polyphenols in humans. Int J Vitam Nutr Res 73: 112-119.
- Shukitt-Hale B (2012) Blueberries and neuronal Gerontology 58: 518-23.
- Li X, Li J, Tang J (2018) A deep learning method for recognizing elevated mature In 2018 33rd Youth Academic Annual Conference of Chinese Association of Automation (YAC)
- Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Salmani ME (2014). Chronic administration of quercetin prevents spatial learning and memory deficits provoked by chronic stress in rats. Behav. Brain Res 270: 196-205.
- Ishola IO, Olubodun-Obadun TG, Ojulari MA, Adeyemi OO (2020) Rutin ameliorates scopolamine-induced learning and memory impairments through enhancement of antioxidant defense system and cholinergic Drug Metab Pers Ther 20200118: 1-9.
- Navarro-Cruz AR, Ramírez Y, Ayala R, Ochoa-Velasco C, Brambila E, et (2017) Effect of chronic administration of resveratrol on cognitive performance during aging process in rats. Oxid Med Cell Longev 2017: 8510761.
- Williams RJ, Spencer JP (2012) Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for alzheimer Free Radic Biol Med 52: 35-45.
- Barrientos RM, Kitt MM, Watkins LR, Maier SF (2015) Neuroinflammation in the normal aging hippocampus. Neuroscience 309: 84-99.
- Deeks SG (2011) HIV infection, inflammation, immunosenescence, and Annu Rev Med 62: 141-155.
- Pallauf K, Gille K, Huebb P, Rimbach G (2013) Nutrition and healthy ageing: calorie restriction or polyphenol-rich “MediterrAsian” diet?. Oxid Med Cell Longev 2013:
- Saied NM, Georgy GS, Hussien RM, Hassan WA (2021) Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin Exp Pharmacol Physiol 48: 337-346.
- Jhang JJ, Lu CC, Ho CY, Cheng YT, Yen GC (2015) Protective effects of catechin against monosodium urate- induced inflammation through the modulation of NLRP3 inflammasome activation. J Agric Food Chem 63: 7343-7352.
- Hayashi Y, Jinnou H, Sawamoto K, Hitoshi S (2018) Adult neurogenesis and its role in brain injury and psychiatric diseases. J Neurochem. 147: 584-594.
- Bondolfi L, Calhoun M, Ermini F, Kuhn HG, Wiederhold KH, et (2002) Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. J Neurosci 22: 515-522.
- Morrens, J, Van Den Broeck W, Kempermann G (2012) Glial cells in adult neurogenesis. Glia 60: 159-174.
- Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest 120: 29-40.
- Michan S, Li Y, Chou MMH, Parrella E, Ge H, et (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30: 9695-9707.
- Shukitt-Hale B, Thangthaeng N, Miller MG, Poulose SM, Carey AN, et al. (2019) Blueberries improve neuroinflammation and cognition differentially depending on individual cognitive baseline status. J Gerontol A Biol Sci Med Sci 74: 977-983.
- Ramli NZ, Yahaya MF, Tooyama I, Damanhuri HA (2020) A mechanistic evaluation of antioxidant nutraceuticals on their potential against age-associated neurodegenerative diseases. Antioxidants 9: 1019.
- Dias GP, Cavegn N, Nix A, do Nascimento Bevilaqua MC, Stangl D, et al. (2012) The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and Oxid Med Cell Longev 2012: 541971.
- Valente T, Hidalgo J, Bolea I, Ramirez B, Anglés N, et al. (2009) A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse J Alzheimers Dis 18: 849-865.
- Flowers A, Lee JY, Acosta S, Hudson C, Small B, et (2015) NT-020 treatment reduces inflammation and augments Nrf-2 and Wnt signaling in aged rats. J Neuroinflammation 12: 1-11.
- Myburgh KH (2014) Polyphenol supplementation: benefits for exercise performance or oxidative stress? Sports Med 44: 57-70.
- Gullo F, Ceriani M, D’Aloia A, Wanke E, Constanti A, et (2017) Plant polyphenols and exendin-4 prevent hyperactivity and TNF-α release in LPS-treated in vitro neuron/astrocyte/ microglial networks. Front Neurosci 11: 500.
- Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, et al. (2016) Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6: 28142.

