New Opportunities in the Synthesis of Monastrol
© 2025 Natalia Ciobanu, Macaev Fliur, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The synthesis of eutectic mixtures based on 3- (carboxymethyl) -1-vinyl-1H-imidazole-3-ium (chloride, bromide, hexofluorophosphate) and thiourea in various ratios (from 2: 1 to 1: 3) was realized, the aggregate state and established catalytic activity for the synthesis of Monastrol, which showed a wide range of pharmacological activity. Recently, interest in the significance of dihydropyrimidines has attracted great interest. Dihydropyrimidines occupy a key place in various biological processes of various body structures that carry vital information. The most effective method currently used for the synthesis of dihydropyrimidines remains the well-studied multicomponent Biginelli reaction. The Biginelli reaction, which is commonly used for the direct preparation of Monastrol and its derivatives, has many advantages over traditional synthetic methods.
Introduction
The growing number of publications and patents on various compounds of this series of this topic only confirm this interest [1]. One of the earliest examples of the use of dihydropyrimidines was as an agent to protect wool from moths [2,3]. In addition, the importance of these compounds in the presence of a heterocyclic system with multifunctional key points having different pharmacological efficacy, which is a goal for study in the future. This direction may be interesting for the pharmaceutical industry [4-6].
Materials and Methods
Three-component and one-pot synthesis under Biginelli reaction conditions makes it possible to obtain monastrol based on the result of the interaction of acetoacetic ester, thiourea, and 3-hydroxybenzaldehyde in the presence of various eutectic catalysts and various solvents and without them [7-9]. From the point of view of environmentally friendly conditions, they have the advantage of many reagents collected in one vessel, which avoids waste from multi-stage purification and the formation of residues [10].
Discussion and Results
A derivative of 3,4-dihydropyrimidine-2(1H)-thiones is the compound ethyl 4-(3-hydroxyphenyl)-6-methyl-2-sulfanylidene- 3,4-dihydro-1H-pyrimidine-5-carboxylate, with the trivial name Monastrol 1 showed biological activity in various directions. Monastrol is a polyfunctional compound. The presence of functional groups in the molecule allows various chemical modifications. The biological role of Monastrol has led to significant interest in its synthesis and is a 3-component one-pot synthesis based on the interaction of acetoacetic ester, thiourea and 3-hydroxybenzaldehyde, which avoids waste from multi- stage purification and the formation of residues. The synthesis is catalyzed by inorganic acids, ionic liquids, eutectic solvents, or under microwave irradiation (Scheme 1) [11-13].
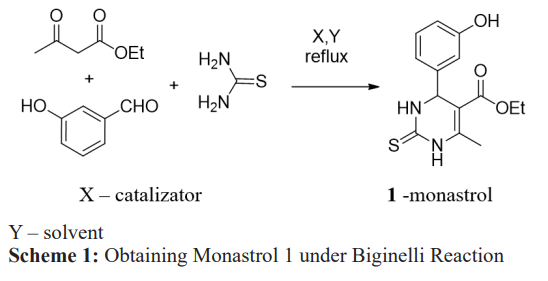
Along with side products, yields of dihydropyrimidines in highly protic or acidic media give low results due to the sensitivity of aldehydes to acid in the case of the classic Biginelli reaction [12]. Therefore, the search for more efficient catalysts is very relevant to this day. From the point of view of “green chemistry”, it is of interest to obtain ionic liquids containing various active groups, such as cyanoethyl, carboxyl, etc., and on their basis to obtain eutectic mixtures with thiourea and to study their catalytic properties in synthesis using the most waste-free and eco-friendly process. monastrol [13-15]. No less important is the fact that ionic liquids and eutectic mixtures in themselves represent substances with the inclusion of biologically active structures. Presented salts 5 are fused with an equimolar amount of thiourea at 103-1050C. Received eutectic mixtures 2,3,4. The compounds are a whitish solid mass with ?m=105-1100? [16-21]. For this purpose, the following were obtained: 3-vinyl-imidazolyl acetic acid hexofluorophosphate: thiourea 2, 3-vinyl-imidazolyl acetic acid bromide: thiourea 3, 3-vinyl-imidazolyl acetic acid chloride: thiourea 4 according to the following scheme 2:
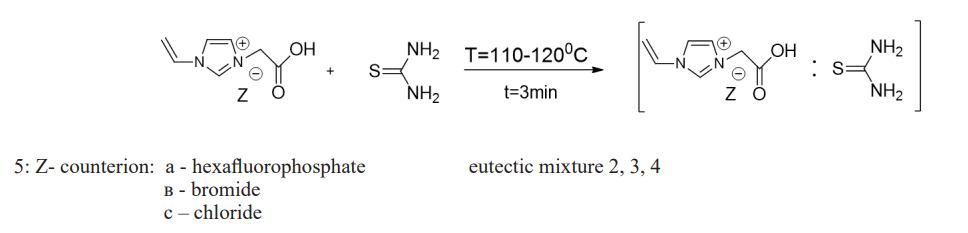

As a result of 2,3,4 as catalysts, a yellowish-gray crystalline substance was obtained as a result, the physicochemical constants of which, including Tm = 182-1840C, indicated the formation of monastrol, the yield of which was 40%. The maximum yield of the product was 79%.
Conclusions
In the synthesis of Monastrol, the goal is to select reagents and test various catalysts that are environmentally friendly, least toxic and financially attractive in order to maximize product yield, reduce reaction time, selectivity and minimize reagent surpluses, formation of by-products, high temperatures, environmental pollution. environment, waste and costs. Catalysis plays a fundamental role in Biginelli synthesis, especially in developing strategies to approach eco-friendly catalytic conditions for further use in the renewable chemical industry.
References
- Kappe C (1993) 100 years of the biginelli dihydropyrimidine In: Tetrahedron 49: 6937.
- Benworth B, Hansen S, Spittle Brian Ch, Derrick P, Zhang Y, et al. (2021) Deep Eutectic Solvents: In: A Review of Fundamentals and Chem. Rev 3: 1232-1285.
- Klein E, DeBonis S, Thiede B (2007) New chemical tools for studying human mitotic kinesin Eg5. In: Bioorganic & Medicinal Chemistry 19: 6474-6488.
- Bose DS, Kumar RK, Fatima L (2004) Efficient and Clean One-Pot Synthesis of 3,4- Dihydropyrimidine-2-(1H)-ones Catalyzed by SrCl2.6H2O-HCl in Solvent or Solvent-Free Conditions. In: KoreaScience Synlett 1-279.
- Deshmukh MB, Anbhule PV, Jadhav SD, Mali AR, Jagtap SS, et al. (2007) Green Expeditious One-Pot Synthesis of 3, 4-Dihydropyrimidin-2(1H)-ones Using a Mixture of Phosphorus Pentoxide-Methanesulfonic Acid at Ambient. In: Temperature.Chem 46: 1545.
- Vdovina S, Mamedov V (2008) New Possibilities of the Classical Biginelli Reaction. Advances in Chemistry 12: 1091-1128.
- Dondoni A, Massi A, Sabbatini S (2002) Improved Synthesis and Preparative Scale Resolution for Racemic In: Tetrahedron Lett 43: 5913.
- Gupta R, Gupta A, Paul S, Kachroo p, Indian J (1995) Synthetic Applications for Microwave Synthesis. In: Heterocyclic Chemistry 34: 151.
- Nesterova E, Grishchenko A (2013) General chemistry with elements of bioorganic chemistry. In: Bulletin of Dnipropetrovsk University “Chemistry” 19: 66-86.
- Grover G, Dzwonczyk S, McMulltn D, Normadinam C, Moreland S, et (1995) Synthesis of New Thiazolopyrimidines Proceeding from 4-Aryl-Substituted 3,4-Dihydropyrimidine- 2(1H)-thiones. In: Pharmacol 26: 289.
- Kundu SK, Majee A, Hajra A (2009) Zinc iodide: a mild and efficient catalyst for one-pot synthesis of aminoindolizines via sequential A3 coupling/cycloisomerization. In: Indian Chem 48: 408.
- Ma JJ, Zang XN, Zhou X, Wang C, Li J, et al. (2007) In: Indian Green Method for the Synthesis of Polysubstituted Chromene Derivatives in Ionic Liquids. Chem 46: 2045.
- Mayer TU (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based In: Science 286: 971-974.
- Mizumo T, Marwanta E, Matsumi N, Ohno N (2004) Allylimidazolium Halides as Novel Room Temperature Ionic In: Chem. Lett 33: 1360-1361.
- Pathak VN, Gupta R, Varshney B (2008) An efficient, inexpensive ‘Green Chemistry’ route to multicomponent Biginelli condensation catalyzed by CuCl2.2H2O-HCl. In: Indian J. Chem 47: 434.
- Ramatchandiran R, Sumathi S, Buvaneswari G (2009) Synthesis, Structure, and Antiradical Activity of New Methano[1,3]Thiazolo[2,3-d][1,3,5]-Benzoxa-Diazocine In: Indian. J. Chem 48: 865.
- Saini S, Kumar S, Sandhu J (2007) Past, present and future of the Biginelli reaction: A critical perspective. In: Indian.
- Chem 46: 1690.
- Saini S, Kumar S, Sandhu J (2007) Synthesis, structure, and antiradical activity of new methano[1,3]thiazolo[2,3-d][1,3,5] benzoxadiazocine In: Indian. J. Chem 46: 1886.
- Shahram E, Shayanfar A (2020) Deep eutectic solvents for pharmaceutical formulation and drug delivery In: Pharmaceutical Development and Technology 7: 779-796.
- Wipf p, Cunningham V (1995) A solid phase protocol of the biginelli dihydropyrimidine synthesis suitable for combinatorial chemistry. In: Tetrahedron Lett 36: 7819-7822.
- Kulakov IV, Talipov SA, Shulgau ZT, Seilkhanov TM (2014) Synthesis, structure and antiradical activity of new derivatives of methano[1,3]-thiazolo-[2,3-d]-[1,3,5]-benzoxadiazocine. In: National Scientific Portal of the Republic of Kazakhstan 10: 604-1613.

