Clinical Analysis of Kidney Disease Combined with Posterior Reversible Encephalopathy Syndrome in Six Children
© 2024 Qingwen Wang, Shuya Zhang, Weilin Xiong, Xiaolei Hu, Ziwei Li, Qingyin Guo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Investigating the clinical and imaging characteristics of Posterior Reversible Encephalopathy Syndrome (PRES) with renal disease among children and improving pediatrician awareness of PRES.
Methods: The clinical presentation, imaging data, treatment, and prognosis of six children diagnosed with kidney disease with PRES at The First Affiliated Hospital of Henan University of Traditional Chinese Medicine between October 2016 and December 2021 were retrospectively analyzed, and relevant literature was reviewed.
Results: Of six children (five boys and one girl) aged from 7 to 14 years, three had Henoch-Schönlein purpura nephritis (HSPN), two had nephrotic syndrome (NS), and one had lupus nephritis (LN). All children had a history of hormone and immunosuppressant therapy. Clinical manifestations of the six children all had convulsions and consciousness disorder. Five had moderate to severe hypertension, two had dizziness and headaches, two had nausea and vomiting, and one had visual disturbances. Cranial magnetic resonance imaging (MRI) of all six patients was characterized by reversible white matter abnormalities, primarily symmetrical. After controlling convulsions, decreasing cranial pressure, and actively controlling the primary disease, all children made a full recovery to their premorbid state.
Conclusions: The main clinical manifestations of PRES include convulsions, consciousness disorder, headache, and visual disturbances. Cranial MRI is an important adjunctive test for the diagnosis of PRES. Children with renal disease treated with hormones and immunosuppressants commonly suffer from PRES. With early diagnosis and aggressive treatment, there is a good prognosis for children with PRES.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinical syndrome with seizures, consciousness disorder, headache, and visual disturbances as primary manifestations, resulting from various aetiologies [1, 2]. This disease can be diagnosed definitively based on history, clinical features, and imaging characteristics. To improve the understanding of pediatricians concerning this disease, the clinical features, imaging characteristics, treatment, and prognosis of six children diagnosed with PRES in our department between October 2016 and December 2021 were analyzed retrospectively.
Data and Methods Clinical Data
Five of the six children in this group were male, and one was female, ranging from 7 to 14 years of age with a mean age of 9.33 years, all having an acute onset. Three children had Henoch- Schönlein purpura nephritis (HSPN), two had nephrotic syndrome (NS), and 1 one had lupus nephritis (LN). Five children had combined moderate to severe hypertension. All six children were treated with hormones and immunosuppressants, which included tacrolimus (FK-506), Triptergium Wilfordii (TW), and mycophenolate mofetil (MMF). Three children were treated with FK-506 (children 2, 4, and 6) for 29 days, four months, and 36 days respectively, with blood concentrations in the normal range. Two children were treated with TW (children 1 and 3) at a dose of > 1.5 mg / (kg⋅d) for eight days and one month, respectively. Child 6 with type IV-G(A) was treated with prednisone combined with FK-506 and MMF, a “multi-target” therapeutic schedule. Three children [1,3,5] were treated with an adequate hormone that was administered orally. The remaining three [2,4,6] took adequate oral hormone, the condition was reduced after remission, and the course was longer. Methylprednisolone was used only in child five and prednisone in all others. (Table 1)
Study Methods
The review was conducted based on the affected children’s clinical manifestations, imaging data, treatment, and results.
Results
Clinical Manifestations
All six children in this group had acute onset of seizures and consciousness disorder. Five children had moderate to severe hypertension, two had dizziness and headaches, two had nausea and vomiting, and one had visual disturbances. All six children had one or more generalized tonic-clonic seizures (Table 1).
Cerebrospinal Fluid (CSF) Examination
Lumbar puncture was performed in five children, and the CSF pressure ranged from 80 to 150 cm H2O. CSF routine and biochemistry were regular, CSF culture exhibited no bacterial growth, and CSF testing for herpes simplex virus, EB virus, and tuberculosis DNA was negative.
Imaging Examination
Cranial magnetic resonance imaging (MRI) was well-established among all six children with PRES. Imaging changes were located in the occipital lobe, cerebellum, and brainstem in the cerebral hemispheres’ posterior part, with simultaneous temporal lobe involvement in three children. Cranial MRI showed symmetrical T1 hypointense or isointense, T2, and FLAIR hyperintense in the fundamental white brain matter. Diffusion-Weighted Imaging (DWI) showed isointense, hypointense, or slightly hyperintense lesions. Cranial Computed Tomography (CT) showed symmetrical hypodense lesions in the posterior white brain matter that predominated. Cranial MRI of six children showed a patchy high signal on the T2WI sequence (Figure 1).
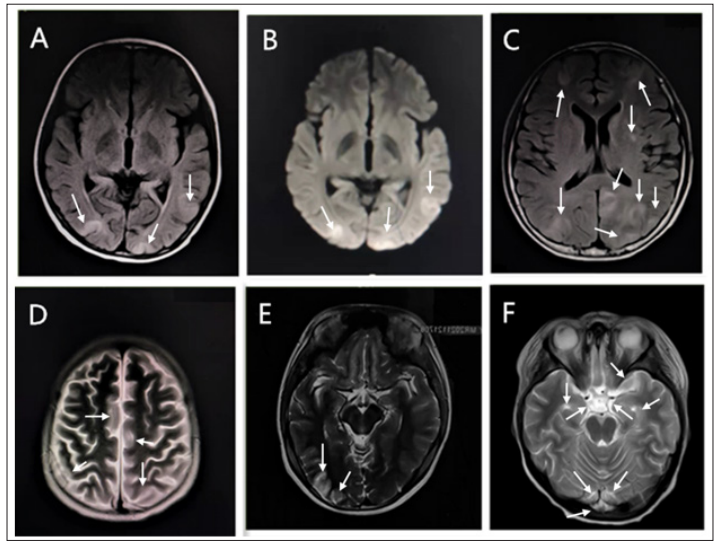
Figure 1: Cranial MRI in Six Children Showed Patchy Hyperintensity on T2WI Sequences.
A.Child 1, B. Child 2, C. Child 3, D. Child 4, E. Child 5, F. Child 6
Treatment and Prognosis
Intracranial pressure in children with PRES was reduced by mannitol for 4 ~ 6 hours every time. Angiotensin-converting enzyme inhibitor (ACEI) or sodium nitroprusside was used for controlling blood pressure. Midazolam or phenobarbital was used for rapid tranquillization and sedation. Electrolyte balance was maintained, and anti-infection and active treatment of primary basic diseases was performed. The neurological symptoms of children can be significantly relieved between two days and one week. Six children with PRES symptoms disappeared within 1 ~ 2 weeks (Table 1). A cranial MRI re-examining found that lesions disappeared entirely in two children and decreased significantly in four.
Table 1: Clinical, Imaging Features and Treatment of Six Patients with PRES
|
Number |
1 |
2 |
3 |
4 |
5 |
6 |
|
Gender |
M |
M |
M |
M |
M |
F |
|
Gender (years) |
8 |
9 |
14 |
8 |
10 |
7 |
|
Basic diseases |
HSPN |
HSPN |
HSPN |
NS |
NS |
LN |
|
premonitory symptoms |
headache |
nothing |
Headache, dizzy and nausea |
nothing |
nothing |
nausea and vomiting |
|
Seizures |
Once, limb twitch, binocular upward gaze |
six times, limb stiffness, head shake, binocular upward gaze, trismus |
three times, limb stiffness and shaking, binocular gaze, trismus, Spitting blood at the mouth |
two times, limb shaking, binoculus eversion, urinary and fecal incontinence |
three times, limb twitch, binoculus eversion |
Once, limb twitch, binocular gaze |
|
State of consciousness |
Loss of consciousness |
Loss of consciousness |
Loss of consciousness |
Loss of consciousness |
Unclear awareness |
Loss of consciousness |
|
neuropsychic symptoms |
anxiety and crying |
Mental confusion, mania, and babbling were present |
visual disturbance, light perception and unclear vision |
Language disorder |
Mental retardation, does not communicate with people, is less mobile |
Talk to yourself, answer questions that are irrelevant, fidgety and crying |
|
blood pressure /mmHg |
117/80 |
144/114 |
135 / 90 |
155/116 |
125/85 |
129/91 |
|
location of lesion |
Bilateral parietal and occipital lobe |
Bilateral parietal and occipital lobe, left temporal lobe and cerebellar hemisphere |
Bilateral frontal, temporal, parietal, occipital lobes, left cerebellar hemisphere, left basal ganglia, and precuneus |
Bilateral parietal and occipital lobe |
Bilateral parietal and occipital lobe |
Bilateral parietal lobe, left thalamus, temporal occipital lobe |
|
CSF examination |
No abnormalities |
No abnormalities |
No abnormalities |
No abnormalities |
Not done |
No abnormalities |
|
Albumin / g•L-1 |
35.8 |
18.0 |
10.6 |
32.5 |
26.3 |
21.4 |
|
24hUTP/mg • 24 h-1 |
2 298 |
4158.7 |
5301. 4 |
1302.7 |
3567 |
4854 |
|
Renal pathology |
HSPN (type IIa) |
HSPN (Focal proliferative type) |
HSPN (Intrafoll-icular hyperplastic type) |
Minimal change glomerulopathy |
not done |
LN type IV-G(A) |
|
Specific medication at onset |
prednisone acetate (25 mg qd), TW (20 mg bid) |
prednisone acetate (20mg; tid), FK506(1.5mg, bid, altogether 90mg), transfusion of plasma, albumin, and human immunoglobul-ins |
prednisone acetate (60 mg qd), TW (20 mg tid), transfusion of plasma and human immunoglobul-ins |
Methylpredniso- lone (16 mg tid), FK- 506 (morning 1 mg, evening 0.5 mg, altogether 125 mg) |
prednisone acetate (15mg, tid) |
prednisone acetate (55 mg qd), FK- 506 (morning 1 mg, evening 0.5 mg, altogether 54 mg), MMF (250 mg bid, altogether 18 g) |
|
Other therapeutic measures |
Reduction of intracranial pressure and blood pressure, sedation and antispasmodic, normal prednisone and dose reduction of TW |
Reduction of intracranial pressure and blood pressure, sedation and antispasmodic, normal prednisone and stop using FK506, anti- infective |
Reduction of intracranial pressure and blood pressure, sedation and antispasmodic, diuresis, Tw halving |
Reduction of intracranial pressure and blood pressure, dose reduction of Methylpredniso-lone, stop using FK506, anti-infection, transfusion of human immunoglobulin-ns |
Reduction of intracranial pressure and blood pressure, sedation and antispasmodic, prednisone halving |
Reduction of intracranial pressure and blood pressure, sedation and antispasmodic, stop using FK506 and MMF, anti- infective, transfusion of human immunoglob-ulins |
M: Male; F: Female; HPSN: Henoch-Schönlein Purpura Nephritis; NS: Nephrotic Syndrome: LN: Lupus Nephritis; CSF: Cerebrospinal Fluid; 24hUTP: 24-Hour Urinary Total Protein;
Discussion
PRES is an encephalopathic syndrome with an acute or subacute onset that is recoverable mainly in the short term, usually in the parietal and occipital lobes, primarily seen in adults [1]. Very few PRES cases in children are reported in the literature [2]. PRES lacks specific clinical manifestations and has a complex etiology. The most common etiology is hypertension, often seen in patients with acutely elevated blood pressure (BP), including hypertensive encephalopathy, eclampsia or preeclampsia, severe kidney disease, infections, autoimmune diseases, tumors, and organ transplants [1, 2]. In addition, immunosuppressants and cytotoxic drugs, including FK-506 and cyclosporine, are important causes of pathogenicity [3].
The potential pathogenesis of PRES is still unknown, and currently, there are four main hypotheses [4]. Vasogenic theory proposes that rapid hypertension leads to excessive vasodilation of cerebral vessels, which exceeds the upper limit of automatic regulation of cerebral vessels and leads to excessive cerebral perfusion [5]. It leads to vasodilation, destruction of the blood-brain barrier (BBB), and vasogenic edema [5-7], with protein and fluid exudation, and then multiple areas of interstitial brain edema [8]. The vertebral basilar artery system’s posterior cerebral circulation, which supplies the brain parenchyma, is more prone to disease, which may be linked to the posterior circulation’s automatic regulation mechanism’s low vascular tension and low sympathetic nerve distribution [7, 9]. Five children with PRES in our cohort had combined moderate to severe hypertension. Consider acute hypertension as one of the etiological factors contributing to the pathogenesis of PRES.
Nevertheless, PRES could still occur in patients with normal or low blood pressure, and fluctuations in blood pressure (rather than maximum blood pressure) might contribute to hyperperfusion [10]. As opposed to that, the cytotoxic theory proposes that both endogenous chemokines in the circulation and exogenous toxins, as well as drugs, can cause vascular endothelium in the microcirculation to be highly vulnerable to inflammatory damage [3, 11], which triggers vascular leakage and edema formation along with activating endothelial cells, which could result into immunogenicity and release of vasoactive substances [2, 8, 12]. According to the neuropeptide theory, when leukocytes are activated, they increase their synthesis and release of vasoconstrictors like prostacyclin, thromboxane A2, and endothelin-1, which causes vasoconstriction and ischemia [13]. Lastly, immunogenicity theory posits the immune system and its interaction with endothelial cells [14]. Increases in inflammation, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6, induce the production of adhesion molecules in the context of systemic inflammatory illnesses (such as SLE, HSP) [3, 14, 15]. Moreover, the release of histamine, nitric oxide, bradykinin, arachidonic acid, and vasopressin increases vascular permeability, leading to consequent fluid leakage [3, 12-14, 16]. TNF - α and IL-1 can also stimulate astrocytes to produce vascular endothelial growth factor (VEGF), weakening tight junctions in the cerebral vasculature [17]. his procedure enhances vasogenic edema and the interaction between leukocytes and endothelial cells. Endothelial dysfunction is caused by these mechanisms (hypertension, azotemia, immune responses) alone or in combination.
The six children in the PRES group all had renal injury, whether primary or autoimmune, and they were all treated with hormones and immunosuppressants, such as FK-506, TW, and MMF. The fifth child in our case series failed to have elevated blood pressure, presenting PRES considering the underlying disease in the affected child and the dysfunction of vascular endothelial cells caused by the high-dose methylprednisolone applied. It has been hypothesized that HSP and SLE, which produce vascular inflammatory alterations that result in endothelial dysfunction in such children, may also be significant causes of PRES due to the discovery of HSP in three children and LN in one. PRES arose from the dysfunction of vascular endothelial cells caused by the underlying disease itself.
A common systemic autoimmune disease is SLE. Nearly every organ and tissue in the body may be affected by SLE [18]. The term “neuropsychiatric systemic lupus erythematosus (NPSLE)” syndromes refer to psychiatric and central, peripheral, and autonomic nervous systems that develop in SLE patients and have no other known causes [18, 19]. NPSLE is among the most potentially disabling and least understood manifestations, with a primarily poor prognosis [19]. Among them, neuropsychiatric syndromes of the central nervous system (CNS) include headache, cognitive dysfunction, convulsive disorder as well as epilepsy, movement disorders, cerebrovascular disease, Psychiatric disorders (anxiety, psychosis, depression), cerebrovascular disease, etc [19, 20]. One of the most common and distinct manifestations of NPSLE, epilepsy, is seen in 10 to 20% of SLE patients [20]. Generalized or localized epilepsy is possible [20]. Patients with SLE linked to epilepsy typically have electroencephalographic abnormalities (60-70%) and often exhibit more gray matter hyperintensities and cortical atrophy on MRI images [21]. Patients with NPSLE may experience transient BBB damage, which results in abnormal permeability between the systemic and cerebral circulations, with subsequent passage of antibodies into the CSF and intrathecal synthesis of antibodies [22]. CSF albumin and IgG gradient measurements, serum indicators of CNS damage, can be used to validate this impairment [18, 22]. n < 1% of patients with SLE, PRES has been linked to the female gender, young age, hypertension, active lupus, renal involvement, lymphopenia, and dyslipidemia [18]. The sixth child had no neuropsychiatric SLE symptoms clinically, including clear consciousness, no response to a question, no cognitive impairment, no severe headache, no movement disorder, no memory loss, and no personality changes. His laboratory tests had been enhancing, without abnormalities in CSF examination. Furthermore, he was already in a mildly active phase of lupus before the convulsions appeared.
FK-506 is a calcineurin inhibitor, and the cytotoxic substances it produces can help promote the release and regulation of endothelin, prostacyclin, and thromboxane A2, directly or indirectly damaging vascular endothelial cells and cause increased permeability of the blood-brain barrier, which induces vasogenic edema and leI ads to PRES [23]. Its neurological side effects often include insomnia, tremor, and paraesthesia [3]. In addition, the mean arterial pressure increased by 35% after FK-506 treatment [4]. Inflammatory mediators such as IL-1 and TNF-α produced by the disease can cause systemic vasoconstriction, increasing the effect of tacrolimus in promoting PRES [3, 4, 14]. There has been an increase in reports of cyclosporine-triggered PRES in recent years. Studies have proven that cyclosporine can result in vascular lesions, has direct toxicity to vascular endothelial cells, and ultimately leads to vascular endothelial injury [3, 4, 6, 23]. Therefore, FK- 506 and cyclosporine-induced vascular endothelial injury are considered essential factors that trigger PRES and risk factors for PRES development. TW is an immunosuppressant widely used in various adult nephropathies but also under challenging nephropathies and immune-related diseases among children [16]. Larger doses were applied to two children, which suggests that large doses for younger children with immune systems that are not well established should alert them to the occurrence of this disease [16]. Although no reports show TW causes adverse effects, including hypertension and convulsions, the combination with hormones should be a concern in PRES. I Its pathogenesis remains unclear, and vascular endothelial injury cannot be excluded, so further clinical observation and research are required. Five children with PRES treated with hormones combined with TW or FK-506, or MMF suggests that immunosuppressants could increase vessel wall permeability, allowing intravascular substances to penetrate the cell interstitium and promoting the occurrence of PRES [3, 16]. In conclusion, the onset of PRES is closely related to the primary underlying disease, hypertension, long-term hormone, and immunosuppressant use, but further study of the exact pathogenesis is required. In the clinic, the blood concentration of immunosuppressive drugs would be regularly detected during treating these diseases in our hospital to treat cytotoxic medications while minimizing their harmful effects on the body.
A review of the hormonal application at disease onset among six children with PRES in our group found high-dose methylprednisolone pulse before ten days of disease onset in child [3]. Moreover, all six children used high doses of long- applied hormones, which suggests that high-dose or long-course application of hormones easily induces the onset of the disease [3].
Children 2 and 3 had severe hypoalbuminemia due to massive proteinuria, hyper edema of both lower limbs with ascites, scrotal edema, oliguria, and anuria. Following a primary treatment of hormones combined with immunosuppressants, IgG decreased, intensive nutritional support treatment was provided, and convulsion episodes occurred after alternating the infusion of plasma, immunoglobulins, and serum albumin, which could be related to the fact that hypertension results in hyperperfusion and damages the BBB [1, 5], immunosuppressants and inflammatory factors [3, 4] damage the vascular endothelium leading to vascular permeability-increasing and following the infusion of blood products, extravasation from the vessels leads to an aggravation of brain edema, which suggests that the infusion of blood products may induce PRES. Studies have shown that when a minimum of two risk factors are present simultaneously, such as hypertension and treatment with FK-506, there is a greater likelihood of severe cytotoxic edema and a worse prognosis than patients who only have hypertension [3].
Common clinical manifestations of PRES are seizures, headaches, visual abnormalities, and impaired consciousness. The clinical manifestations of the cases in this study were seizures in all six children (100%), disturbance of consciousness in all six children (100%), headaches in two children (33.33%), nausea and vomiting in two children (33.33%) and visual abnormalities in one child (16.67%). These symptoms were all completely resolved within days or weeks [1, 19]. However, as the clinical manifestations of PRES lack specificity, most pediatricians are unaware of the characteristics of this disease, potentially leading to misdiagnosis and missed diagnosis. In addition, if rational treatment is not provided at the early stage of the disease, vasogenic edema may progress to cytotoxic edema and, ultimately, cerebral infarction or cerebral hemorrhage, which can lead to permanent brain damage.
The more typical imaging changes of PRES are essential for disease diagnosis, which is mainly based on the symmetric involvement of the white matter in the posterior areas of the bilateral cerebral hemispheres, sparing the frontal and temporal lobes and other atypical areas such as the cerebellum, brainstem, basal ganglia, and other grey matter [15, 24]. CT is often the first screening option used in an emergency setting and shows bilateral parieto-occipital patchy hypodense opacities, but the diagnosis can easily be missed [24]. MRI is the most effective ancillary examination for PRES diagnosis [24]. MRI can appear isointense or hypointense on T1 WI sequences and patchy hyperintense on T2 WI sequences. DWI appears isointense or hypointense with elevated apparent diffusion coefficient (ADC) values and patchy hyperintense shadowing that is suggestive of vasogenic edema, which is different from cytotoxic edema and may reflect PRES prognosis [25].
Treatment measures for PRES mainly include dehydration for decreasing cranial blood pressure, controlling blood pressure, startling, the maintenance of electrolyte balance, resistance to infection, and removing or reducing precipitating factors [6]. Controlling blood pressure is an excellent benefit for the prevention of worsening of the disease, generally through the increased use of calcium channel blockers (CCBs) and angiotensin-converting enzyme inhibitors (ACEIs) in addition to central antihypertensive agents [2, 6]. The elimination of aetiological treatment while attempting to use the treatment of the primary disease is a vital aspect of PRES treatment [18, 23]. The neurological symptoms of these youngsters were entirely resolved after two weeks of reducing prednisone dosage or halting immunosuppressive drugs and symptomatic support for primary disorders and determining the immunosuppressive drug concentration in the blood. The re-examination of the brain MRI displayed that the lesions had been significantly reduced or disappeared. Three months later, the re-examination of the brain MRI displayed that the lesions were completely eliminated.
Clinically, children with renal or autoimmune diseases or patients with the massive long-term application of hormones and simultaneous use of immunosuppressants and other high-risk factors must control their blood pressure aggressively and be vigilant for PRES when hypertension appears [3, 15]. In addition to regularly monitoring the blood concentration of cytotoxic drugs, MRI should be performed as early as possible to provide a definite diagnosis for those with the sudden onset of severe headaches and other symptoms. Early diagnosis and treatment are crucial for the prognosis of this disease [3].
The area for improvement in our study is that we may have missed mild cases. Mild cases may present with only visual changes, nausea, vomiting, or headache [26]. PRES may be more aggressive in children with kidney disease due to structural and developmental differences between the pediatric and adult CNS. While treating kidney and autoimmune diseases, our pediatric department has started taking corresponding symptomatic treatments such as lowering blood pressure and reducing hormones or immunosuppressants when mild visual changes, Nausea, Vomiting, or headache occur. When considering the medical costs for the child’s family burden, imaging examination is no longer done after the mild symptoms have significantly decreased or disappeared; imaging examination will only be performed if mild symptoms persist or worsen. Since the treatment eliminates the trigger, maintaining a low threshold for diagnosis is essential for a cure.
In conclusion, PRES is a delicate reversible process that can be alleviated or eradicated in the short term and wholly resorbed by imaging lesions if detected, correctly diagnosed, and treated promptly. However, if it is delayed, this can lead to clinically irreversible sequelae or even death.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Qingwen Wang is responsible for writing the whole article. Thank Qingyin Guo for his guidance, thanks to Shuya Zhang, Weilin Xiong, Xiaolei Hu and Ziwei Li for revising the article.
Funding
The Natural Science Foundation of Henan Province (2123004103); Project of Henan Provincial Administration of Traditional Chinese Medicine (2019zy2113; 20-21zy1048); Particular Project for Establishing a “Double First Class” Scientific Research in Traditional Chinese Medicine in Henan Province (HSRP- DFCTCM-2023-17; HSRP-DFCTCM-2023-4).
References
- Gewirtz AN, Gao V, Parauda SC, Robbins MS (2021) Posterior Reversible Encephalopathy Syndrome. Curr Pain Headache Rep 25: 19.
- Bilir OA, Dikme G, Malbora B, Evim MS, Sivis ZO, et al. (2021) Posterior Reversible Encephalopathy Syndrome in Childhood Hematological/Oncological Diseases: Multicenter J Pediatr Hematol Oncol 43: e462-e465.
- Kaur G, Ashraf I, Peck MM, Maram R, Mohamed A, et al. (2020) Chemotherapy and Immunosuppressant Therapy- Induced Posterior Reversible Encephalopathy Syndrome. Cureus 12: e11163.
- Liman TG, Siebert E, Endres M (2019) Posterior Reversible Encephalopathy Syndrome. Curr Opin Neurol 32: 25-35.
- Byrom FB (1954) The Pathogenesis of Hypertensive Encephalopathy and Its Relation to the Malignant Phase of Hypertension; Experimental Evidence from the Hypertensive Lancet 267: 201-211.
- Ghali MGZ, Davanzo J, Leo M, Rizk E (2019) Posterior Reversible Encephalopathy Syndrome in Pediatric Patients: Pathophysiology, Diagnosis, and Management. Leuk Lymphoma 60: 2365-2372.
- Hansson HA, Johansson B, Blomstrand C (1975) Ultrastructural Studies on Cerebrovascular Permeability in Acute Hypertension. Acta Neuropathol 32: 187-198.
- Bartynski WS (2008) Posterior Reversible Encephalopathy Syndrome, Part 2: Controversies Surrounding Pathophysiology of Vasogenic AJNR Am J Neuroradiol 29: 1043-1049.
- Schwartz RB, Mulkern RV, Gudbjartsson H, Jolesz F (1998) Diffusion-Weighted Mr Imaging in Hypertensive Encephalopathy: Clues to Pathogenesis. AJNR Am J Neuroradiol 19: 859-862.
- Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, et al. (2012) Blood Pressure Fluctuations in Posterior Reversible Encephalopathy J Stroke Cerebrovasc Dis 21: 254-258.
- Lum H, Qiao J, Walter RJ, Huang F, Subbaiah PV, et al. (2003) Inflammatory Stress Increases Receptor for Lysophosphatidylcholine in Human Microvascular Endothelial Cells. Am J Physiol Heart Circ Physiol 285: h4786-h4789.
- Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J (2006) Posterior Reversible Encephalopathy Syndrome in Infection, Sepsis, and Shock. AJNR Am J Neuroradiol 27: 2179-2190.
- Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, et (2003) Highly Enhanced Permeability of Blood-Brain Barrier Induced by Repeated Administration of Endothelin-1 in Dogs and Rats. Pharmacol Toxicol 92: 21-26.
- Chen Z, Shen GQ, Lerner A, Gao B (2017) Immune System Activation in the Pathogenesis of Posterior Reversible Encephalopathy Syndrome. Brain Res Bull 131: 93-99.
- Ollivier M, Bertrand A, Clarencon F, Gerber S, Deltour S, et (2017) Neuroimaging Features in Posterior Reversible Encephalopathy Syndrome: A Pictorial Review. J Neurol Sci 373: 188-200.
- Song CY, Xu YG, Lu YQ (2020) Use of Tripterygium Wilfordii Hook F for Immune-Mediated Inflammatory Diseases: Progress and Future Prospects. J Zhejiang Univ Sci B 21: 280-290.
- De Medeiros FC, Rocha Sousa BM, Cruz Santos DN, Novais Matias Sion G, Fontes Alves C (2022) Posterior Reversible Encephalopathy Syndrome as the First Clinical Manifestation of Lupus Nephritis. Acta Neurol Belg 122: 219-221.
- Carrion Barbera I, Salman-Monte TC, Vilchez-Oya F, Monfort J (2021) Neuropsychiatric Involvement in Systemic Lupus Erythematosus: A Review. Autoimmun Rev 20: 102780.
- Valdez Lopez M, Aguirre Aguilar E, Valdes Ferrer SI, Martinez Carrillo FM, Arauz A, et al. (2020) Posterior Reversible Encephalopathy Syndrome: A Neuropsychiatric Manifestation of Systemic Lupus Autoimmun Rev 20: 102739.
- Appenzeller S, Pereira DR, Julio PR, Reis F, Rittner L, et (2022) Neuropsychiatric Manifestations in Childhood-Onset Systemic Lupus Erythematosus. Lancet Child Adolesc Health 6: 571-581.
- Rezgui A, Ghannouchi N, Gabbouj A, Anoun J, Karmani M, et al. (2017) Seizures in Patients with Systemic Lupus Erythematosus. Rev Med Liege 72:101-105.
- Deijns SJ, Broen JCA, Kruyt ND, Schubart CD, Andreoli L, et al. (2020) The Immunologic Etiology of Psychiatric Manifestations in Systemic Lupus Erythematosus: A Narrative Review on the Role of the Blood Brain Barrier, Antibodies, Cytokines and Chemokines. Autoimmun Rev 19: 102592.
- Stabouli S, Chrysaidou K, Kupferman JC, Zafeiriou DI (2019) Neurological Complications in Childhood Nephrotic Syndrome: A Systematic Eur J Paediatr Neurol 23: 384-391.
- Mai H, Liang Z, Chen Z, Liu Z, Xu Y, et al. (2021) Mri Characteristics of Brain Edema in Preeclampsia/Eclampsia Patients with Posterior Reversible Encephalopathy BMC Pregnancy Childbirth 21: 669.
- Li K, Yang Y, Guo D, Sun D, Li C (2020) Clinical and Mri Features of Posterior Reversible Encephalopathy Syndrome with Atypical Regions: A Descriptive Study with a Large Sample Size. Front Neurol 11: 194.
- Onder AM, Lopez R, Teomete U, Francoeur D, Bhatia R, et (2007) Posterior Reversible Encephalopathy Syndrome in the Pediatric Renal Population. Pediatr Nephrol 22: 1921-1929.

