Assessment of Pesticide Residues in Vegetables Commonly Consumed in The Democratic Republic of Congo (DRC): Inadequate Agricultural Practices and Potential Impacts for Public Health
© 2023 P M Ndelo, L M Mputu, Y Nuapia, J D P, Ndelo, J K Tuakuila, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pesticides residues in food pose a serious risk to children and adults consuming pesticide-contaminated food. The aim of present study was to assess pesticide residues in vegetables in the Kinshasa and Lubumbashi cities of the Democratic Republic of Congo (DRC). The levels of three pesticide residues were determined by gas chromatography coupled with electron capture detector (ECD) or mass spectrometer-time of flight detector (GC–ECD or GC-MS-TOF) in 96 samples of four vegetables amaranth, spinach, sorrel and sweet potato-leaves purchased from wholesale markets. The Dichlorodiphenyltrichloroethane with its metabolites (3-DDTs), endosulfan and malathion residues were found in 100% of the vegetable samples from Lubumbashi and in 62.5% to 87.5% of all vegetable samples from Kinshasa. Risks were mainly associated with the residues of DDTs pesticides in vegetables. The HQ and HI estimations revealed a serious potential risk to consumers, children particularly. Due to multiple pesticide residues exceeding the MRLs for single residue levels, the consumers are exposed to pesticides, heavily in Lubumbashi. Due to increasing trend in pesticide use, continuous monitoring of pesticide residues in vegetables and other food is recommended in order to develop the base line data on which pesticide regulations could be enhanced in DRC.
Introduction
Pesticides are chemical substances, which are commonly used in modern agriculture practices to protect the crops from different pests and diseases producing food [1]. Pesticides residues in food pose a serious risk to children and adults consuming pesticide- contaminated food. In international monitoring programs to protect people from the toxic effects of exposure to pesticides, the European union (EU), Food and Agricultural Organization/ World Health Organization (FAO/WHO) and US Environmental Protection Agency (EPA) have established limits on pesticide residues in foods [2, 3]. The highest concentration of a pesticide residue (refereed as a maximum residue level -MRL) is legally accepted in or on food when pesticides are applied correctly (based on Good Agricultural Practices -GAP) [4]. The presence of residues with level exceeding MRLs should be less than 20% in most national monitoring programs and interpreted as violation of GAP. The risk assessment for long-term and short-term exposure must be done for all pesticides detected to ensure consumer’s health protection. The hazard quotient (HQ) is a tool that is used for the assessment of health risk (HR) due to human exposure to a single pesticide [5]. The hazard index (HI) is used for assessing the cumulative risk index of pesticides that cause the same toxic effects in tissues, organs and physiological systems [6,7].
The inadequate application of pesticides may produce large quantities of residues in the environment and easily enter into the human food chain through plants, creating a potentially serious health hazard [8-10]. Hence, each pesticide has different properties and toxicological effects. For example, many of the older, such as dichlorodiphenyltrichloroethane (DDT), endosulfan and malathion can remain for years in soil and water. Due to its lipophilic properties and high resistance to biodegradation, DDT is recognized as a persistent environmental pollutant and endocrine disruptor [10,11]. Epidemiologic studies revealed either positive or negative associations between exposure to DDT and tumor development, but there has been no clear evidence that DDT causes cancer in humans [12]. Endosulfan is an organochlorine compound that is used as an insecticide and acaricide. This colorless solid has emerged as a highly controversial agrichemical due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor [9]. Malathion is one of the oldest organophosphate insecticides with a broad spectrum of agricultural and public health applications. It has been suggested that a significantly lower pregnancy rate or live birth rate can be evoked with a mixture of organophosphate pesticides, including malathion [8-13]. Malathion is classified as probably carcinogenic to humans (Group 2A) [14]. The primary route of exposure for the general public is through ingestion of food previously treated with pesticides and drinking water contaminated with agricultural runoff [8-10]. Other routes of exposure include dermal contact and inhalation during the use of products containing pesticides or during activity in areas previously treated with these pesticides. In the Democratic Republic of Congo (DRC) as in most developing countries, pesticides have been used for many decades in agricultural practices, for instance in the cultivation of vegetables [15]. Since the year 2013, the Ministry of Agriculture of DRC has approved the import, sale, and use of several pesticides including endosulfan, dithiocarbamate, rayasansulfan, rhodiatox, delthaméthrine and metyldor for urban agricultures. Consequently, pesticides are extensively applied for pests’ control to improve yield of urban agriculture practices for the production of vegetables and fruits. On other hand, there is no control or application of regulation concerning the use of these substances [16]. In Kinshasa, the capital of the DRC, the urban population has increased from 300.000 in 1960 to more than 10. 000,000 inhabitants in 2010. This sharp increase in the urban population leads to several challenges, including food security. To meet these challenges, poor families in cities are resorting to urban and peri-urban agriculture, including market gardening [17]. In Lubumbashi, the second largest urban city, market gardening, whether directed towards self-consumption or sale, is part of the livelihood of many households [18]. Recent surveys of phytosanitary practices in these two cities have revealed a misuse of pesticides on the part of producers, not respecting the doses or frequencies of application on crops. And the pesticides used were mostly banned and withdrawn from the market. For example, DDT (dichloro-diphenyl trichloroethanes), used mainly in Kinshasa [19]. The misuse of these products would inevitably lead to the presence of residues in crop products, which can be toxic to consumers. Therefore, the aim of present study was to assess pesticide residues in vegetables in the Kinshasa and Lubumbashi cities of DRC with a view to ensure consumer’s health protection.
Samples: Materials and Methods
Pesticide residues were determined in 96 vegetable samples collected from local wholesale markets of Kinshasa, Lubumbashi, and transported to laboratory in South Africa. according to standard sampling procedure [20,21]. The vegetable samples surveyed from June 2020 to January 2021 were amaranth (Amaranthus viridis), spinach (Spinacia oleracea), sorrel (Rumex acetosa), sweet potato- leaves (Ipomoea batatas). All vegetable samples were dried in herbarium away from the sun’s rays and were stored at 400C prior to extraction procedure.
Chemical Standards and Reagents
Pesticide standards (Endosulfan, DDT,4,4’-DDE. 4,4’-DDD and Malathion) were purchased from Merck (Johannesburg, South Africa) with purity between 95%. Anhydrous sodium sulphate, acetonitrile, magnesium sulphate monohydrate, sodium chloride and bondesil primary/secondary amine (PSA) were also from Sigma-Aldrich. The stock solutions of 5 mg. L-1 were prepared in hexane/toluene (50/50) mixture and stored in freezer at 40°C. The concentration of 1.5 and 10.0 µg. L-1 were used as working solution for calibration.
Extraction Procedure
The Quenchers extraction method was done using the modified procedure reported by Rawn et al. 2010 [22]. Homogenized samples with no pesticides detected on previous occasions were used for recovery studies, and for the preparation of matrix- matched standards for calibration. The homogenized samples were spiked with 1.5 and 10 mg. L-1 of a standard mixture of seventeen organochlorines. The spiked samples were allowed to stand for 30 min. 10 g of homogenized food sample was put in a 50 mL Teflon tube. Then 10 mL of acetonitrile was added, and the sample was shaken strongly for 1 minute. This was followed by salting-out step with additions 1.5 g sodium chloride and 3 g of anhydrous magnesium sulphate into the tube and the mixture was shaken vigorously for 1 minute and then centrifuged. After centrifuge, 6.5 mL of organic supernatant was transferred into the polypropylene centrifuge tube to clean-up with 1.65 g anhydrous magnesium sulphate and 27.5 g primary/secondary amine (PSA). The solution was centrifuged for 5 min and filtered using a 0.45 mm PTFE and injected in the GC -ECD and/or GCxGC/TOFMS for analysis [6, 23].
GC–ECD or GC-MS-TOF analysis
The extracts were analyzed using GC- ECD or GC-MS under conditions as follows: The GC conditions and the detector response were adjusted to match the relative retention times and response. The conditions used for the analysis were: capillary column coated with ZB-5 (30 m x 0.25 mm, 0.25 mm film thickness). Nitrogen (99.999%) was used as carrier gas flowing at 1.2 mL. The oven temperature was programmed from 60°C (1 min) to 180°C at a rate of 30°C. min-1, 180°C (3 min) to 300°C at a rate of 3°C. min-1. The temperature of the injector operating in split less mode (volume injected 1 mL) was held at 300°C and electron-capture detector temperature was 250°C. The conditions of the mass spectrometer were as follows: transfer line temperature 290 C; ion source temperature 2500C and multiplier voltage 1450V. A programmed temperature vaporization injector operating in solvent-split mode was employed. The volume injected was 9 mL, split flow 50 mL.min-1 and injection time: 0.50 min, injection flow: 100 mL.min-1. The oven temperature program was as follows: initial temperature of 50°C increased to 150°C at 10°C.min-1 followed by an increase to 300°C at a rate of 5°C.min-1. He was used as carrier gas flow rate of 1 mL.min-1. Ion trap mass detection was operated in full scan mode from 50 to 500 amu. The GC- MS-TOF was used to confirm the identification of compounds in samples.
Health Risk Assessment Health risk estimation
Data on pesticide residues level were compared with recommended maximum residue limits (MRLs) set by [23-25]. The non-carcinogenic and carcinogenic health risk estimates for each pesticides residues in leafy vegetables were computed using three basic standard indices: The Estimated Daily Intake (EDI), Cancer Benchmark Concentration (CBC) and the Health Risk Index (HRI). Estimated Daily Intakes (EDI) of a pesticide residue and food consumption assumption were used to determine long term health risks to consumers. The EDI was obtained by multiplying the mean residual pesticide concentration (mg. kg-1) in the vegetable of interest and the vegetable consumption rate (kg. d-1) and dividing by body weight [26-28]. Consumption rate for vegetable in DRC is 120 g/person/day [20].
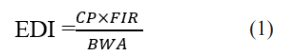
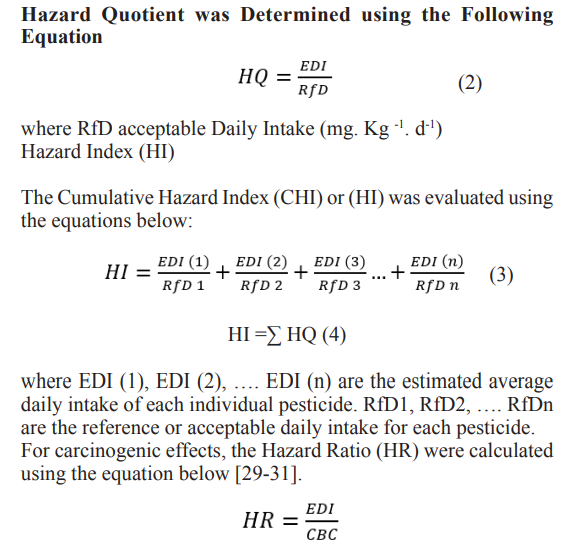
where CP, FIR and BWA represent mean pesticide concentration in vegetable (mg .kg -1), food consumption rate (kg. d-1), and average body weight, respectively. The non-carcinogenic health risk was assessed by calculating the health risk index (HI) which was evaluated by dividing the EDI by their corresponding values of ADI with an assumption of average adult’s body weight of 60 kg while children considered to have an average body weight of 16.7 kg [27]. It was also assumed that absorption and bioavailability rates are 100%. When the health risk index > 1, the food involved is considered a risk to the consumers; when the index < 1, the food involved is considered acceptable [26,27].

Statistical Analysis
The descriptive analysis of data was performed using Minitab 16 Software with level of significance maintained at 95%. The percentage (%) recoveries together with limit of detection (LOD) and limit of quantification (LOQ) were also determined [20].
Results and Discussion Pesticide Residues in Vegetables
Figure 1 and Figure 2 display the rate of detection and comparative levels of pesticide residues found in this study. The 3-DDTs, endosulfan and malathion residues were found in 100% of the vegetable samples from Lubumbashi. The same pesticide residues were observed in 62.5% to 87.5% of all vegetable samples from Kinshasa. Their concentrations were generally higher in Lubumbashi than in Kinshasa. The higher levels of pesticide residues in vegetables from Lubumbashi can be since pesticides were extensively used in the past in Lubumbashi compared to their use in Kinshasa. This detection of pesticide residues in vegetables is far higher than in a similar study conducted in Tanzania. which reported that pesticide residues were detected in 29% of all samples analyzed and comparable to those in countries such as Burkina Faso Kuwait Tanzania and Senegal [33-37]. It
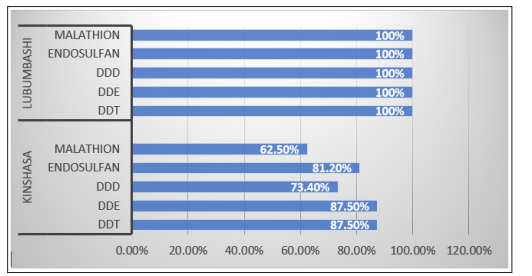
Figure 1: Pesticide residues frequency in lubumbashi and kinshasa studied vegetables
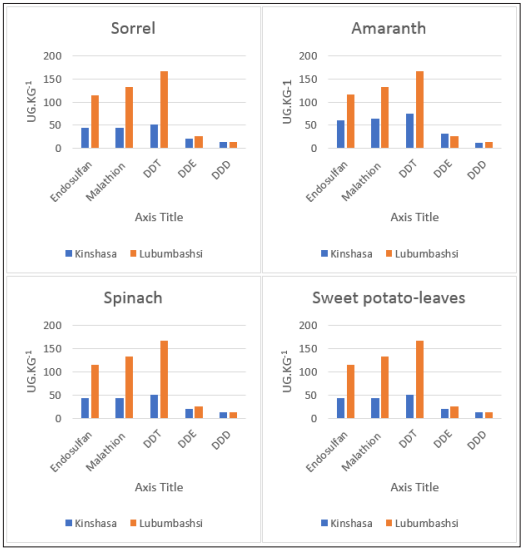
Figure 2: Comparative levels pesticides in vegetables purchased in kinshasa and lubumbashi
The concentrations of pesticide residues discovered are presented in Table 1 and were trending as DDTs > malathion > endosulfan. The sorrel vegetables had the highest concentration of endosulfan 0.13 (0.09- 0.26) mg.kg-1 and malathion 0.15 (<0.01-0.29) mg.kg- 1from Lubumbashi while the lowest levels were 0.03 (<0.01- 0.19) mg.kg-1and 0.01 (<0.01-0.08) mg.kg-1respectively from Kinshasa. The highest 3-DDTs, concentration was observed in spinach from Lubumbashi, with mean concentration (range) of 0.30 (0.16-0.32) mg.kg-1. Indeed, most of the DDT is slowly degraded to DDE and DDD by microorganisms; half of the DDT in soil is degrades between 2 and 15 years, depending on the type of soil [10]. Specific ratios of parent/metabolites of organochlorine pesticides compounds have been widely used to identify past and present input application into the environment [25-32]. If the ratio of DDT/DDE is found to be more than 1, then it indicates recent used of DDT compounds while DDE/DDT. The ratio of DDT/ DDE from Kinshasa was 2.2 for sorel samples, 2.4 for sweet potato leaves, 2.3 for amaranths, 2.5 for spinach. The ratios are equivalent for all crops analyzed. The DDT/DDE ratio from Lubumbashi was 5.8 for sorrel samples, 6.3 for sweet potato leaves, 6.3 for amaranths, and 6.0 for spinach. In consequent, The DDT/DDE ratios from Lubumbashi were almost 3 times higher than those from Kinshasa. This provides information on a more recent use of DDT in Lubumbashi. This observation is in the same line that Yanick’s study which indicated a recent application of DDT in vegetables from DRC and Johannesburg vegetables [20].
Levels of Pesticide Residues Versus Mrls
Pesticide residues were found in 81% of the amaranth and 93% of the sorrel or spinach samples from Lubumbashi samples exceeded the MRLs (Table 1). In the light of increasing levels, noticeable greater level of pesticide residues was observed in sweet potato-leaves samples in which 84% samples exceeded the MRL values of endosulfan, followed by sorrel (75%). In Kinshasa, 30% samples of the amaranth and 14% samples of the sorrel or spinach exceeded the MRL values of DDTs, while these rates exceeded the MRL values of endosulfan in 69% samples of the spinach and 21% samples of the sweet potato-leaves. This is close to the results by Amoah study in Ghana where most of the residues recorded exceeded the maximum residue limit for consumption of vegetables in Ghana’s urban markets [39] and in Hyderabad, Pakistan [40]. The negligence or non-availability of proper guidance about pesticide application may be a principal reason, which may lead to contamination of vegetables with pesticide residues, mainly in most developing countries [34-40].
Table 1: Mean concentrations and MRLs of pesticides residues
in vegetables from Kinshasa and Lubumbashi.
|
|
|
Sorrel |
Sweet potato-leaves |
Amaranth |
Spinach |
||||
|
Pesticide |
Range |
mean |
Range |
Mean |
Range |
Mean |
Range |
Mean |
|
|
Kinshasa |
|
||||||||
|
Endosulfan |
0-187,2 |
29,3± 48 |
0-187,2 |
45,1±60,6 |
0-160,7 |
59,8±44,8 |
0-367,5 |
103,7±100,3 |
|
|
Malathion |
0-78,2 |
13,6±20,8 |
0-228 |
43,9±38,6 |
0-390,6 |
64±104 |
0-417,3 |
102,9±123,1 |
|
|
DDT |
0-111,6 |
28,5±27,6 |
0-205,2 |
51,6±50,8 |
0-269,8 |
74,5±65,1 |
0-310,6 |
117,8±102,3 |
|
|
DDE |
0-59,2 |
12,8±14,4 |
0-81,4 |
21,2±21,6 |
0-140,1 |
32,5±35,7 |
0-149,7 |
47,2±42,9 |
|
|
DDD |
0-36,7 |
6,3±9,3 |
0-36,7 |
14,9±17,6 |
0-20,1 |
12,6±9,4 |
0-71,9 |
20,8±19,2 |
|
|
Lubumbashi |
|
||||||||
|
Endosulfan |
21,1-259 |
134,7±77,9 |
23,7-296,1 |
116,1±83,2 |
43,7-256,7 |
116,6±77,3 |
33,1-117,5 |
73,5±34,2 |
|
|
Malathion |
25,6-294 |
153,8±87,9 |
28,4-335,9 |
132,6±93,9 |
51,8-293,6 |
133,3±87,2 |
39,1-134,4 |
82,0±36,5 |
|
|
DDT |
30,5-369,9 |
192,6±111,2 |
34,1-422,2 |
166±118,7 |
62,7-369,4 |
166,7±110,3 |
47,5-168,5 |
104,2±45,3 |
|
|
DDE |
3-71,3 |
33±24,5 |
2,9-83 |
26,2±26,2 |
3,4-71,2 |
26,4±24,3 |
0,1-61,5 |
17,5±31,4 |
|
|
DDD |
0,9-36 |
17,6±11,5 |
1,2-41,4 |
14,9±12,2 |
4,2-35,9 |
14,9±11,4 |
2,6-91,6 |
17,5±34,8 |
|
Human Health Risk Assessment
Pesticides have high toxicity, and their toxic effects on humans are recognized as either acute or chronic, the latter resulting from a long-term exposure to low doses or regular intake of pesticide residues from food. Therefore, the health risks of pesticides were focused on oral ingestion. This is because ingestion remains a major risk among the various routes of exposure [8-10]. The human risks of pesticide residues were assessed in Table 2 and Table 3 for both adults and children, respectively [41]. These tables assessed adult and children dietary exposure using HQ and HI equations for the identified pesticides. In adults, HI < 1 was observed for all vegetable samples from Kinshasa indicating less risk of non-carcinogenic health effects, while Adeleye et al in Nigeria reported HI > 1 that could pose systemic health effect for adult consumers of the vegetable. In Lubumbashi, HI > 1 in all sorrel samples but ranged from 0.6 to 0.9 for other vegetable samples. It should be noted that for each HI value, DDT had the greatest estimated risk, as reported in Kinshasa by Yanick et al. [20]. It could be explained by inappropriate abusive treatments without respecting safety recommendations in farms. In contrast, the estimated HI values for children showed potential chronic health risks as HQ > 1 for all vegetables consumed in both cities, except sorrel from Kinshasa. Of all of the HQs, the highest factor was in spinach (HQ = 2.8), followed by amaranth (HQ = 1.83) and sweet potatoes leaves (HQ = 1,34) showing the highest risks of dietary exposure in Kinshasa. In Lubumbashi, the HQs in children were higher and ranging from 2.1 to 3.7. This finding was reported by Salma et al. indicating that contaminated vegetables might pose a potential threat to children’s health [42]. However, Adeoluwa et al. reported that HI > 1 in the vegetable for both, children and adults [41]. It is important to mention that the diet of the Congolese does not only contain vegetables. These are often accompanied by fish or meat. Considering the abundant use of pesticides in the DRC, including the very persistent DDT used both in agriculture and in mosquito control [43]. There is also concern about the high health risk among adults in the DRC. Considering the abundant use of pesticides in the DRC, including the very persistent DDT used both in agriculture and in mosquito control, we can fear a risk of chronic non-carcinogenic poisoning and / or carcinogenic risk of the populations of the DRC. This has been demonstrated by Yanick’s research on the risk assessment of organochlorines in fish and meat samples. He reported in these conclusions that DDT poses a chronic health risk problem in the DRC.
Table 2: Adult Estimated dose (mg day-1 kg-1),
reference dose (mg day-1 kg-1), and combined risk of multiple pesticides (HI) in vegetables from Lubumbashi and Kinshasa markets
|
|
|
|
Sorrel |
Sweet potato-leaves |
Amaranth |
Spinach |
||||
|
|
|
|
EDI |
HQ |
EDI |
HQ |
EDI |
HQ |
EDI |
HQ |
|
|
|
RfD |
|
|
|
|
|
|
|
|
|
Kinshasa |
|
|
|
|
|
|
|
|
|
|
|
|
Endosulfan |
0,006 |
0,00006 |
0,01 |
0,000093 |
0,015 |
0,00012 |
0,0206 |
0,00021 |
0,0358 |
|
|
Malathion |
0,02 |
0,000028 |
0,0014 |
0,000091 |
0,004 |
0,00013 |
0,0066 |
0,00021 |
0,0106 |
|
|
DDT |
0,0005 |
0,000059 |
0,118 |
0,0001 |
0,214 |
0,00015 |
0,309 |
0,00024 |
0,489 |
|
|
DDE |
0,0005 |
0,000026 |
0,053 |
0,000044 |
0,088 |
0,000067 |
0,134 |
0,000097 |
0,195 |
|
|
DDD |
0,0005 |
0,000026 |
0,026 |
0,00003 |
0,061 |
0,000026 |
0,052 |
0,000043 |
0,086 |
|
|
|
|
|
0,209 |
|
0,384 |
|
0,523 |
|
0,611 |
|
∑HI |
|
|
|
|
|
|
|
|
|
|
|
Lubumbashi |
||||||||||
|
|
Endosulfan |
0,0006 |
0,00027 |
0,045 |
0,00024 |
0,04 |
0,00024 |
0,04 |
0,00015 |
0,0254 |
|
|
Malathion |
200 |
0,00031 |
0,015 |
0,00027 |
0,0137 |
0,00027 |
0,0138 |
0,00017 |
0,0085 |
|
|
DDT |
0,0005 |
0,0004 |
0,799 |
0,00034 |
0,689 |
0,00034 |
0,692 |
0,00021 |
0,432 |
|
|
DDE |
0,0005 |
0,000068 |
0,137 |
0,000054 |
0,108 |
0,000054 |
0,109 |
0,000036 |
0,072 |
|
|
DDD |
0,0005 |
0,000036 |
0,073 |
0,00003 |
0,061 |
0,00003 |
0,061 |
0,000036 |
0,072 |
|
∑HI |
|
|
|
1,07 |
|
0,91 |
|
0,91 |
|
0,61 |
Table 3: Children Estimated dose (mg day-1 kg-1), reference dose
(mg day-1 kg-1), and combined risk of multiple pesticides (HI) in vegetables from Lubumbashi and Kinshasa markets
|
|
|
|
Sorrel |
Sweet potato-leaves |
Amaranth |
Spinach |
||||
|
|
|
|
EDI |
HQ |
EDI |
HQ |
EDI |
HQ |
EDI |
HQ |
|
|
|
RfD |
|
|
|
|
|
|
|
|
|
Kinshasa |
|
|
|
|
|
|
|
|
|
|
|
|
Endosulfan |
0,006 |
0,00021 |
0,035 |
0,00032 |
0,054 |
0,00043 |
0,072 |
0,00075 |
0,125 |
|
|
Malathion |
0,02 |
0,000098 |
0,0049 |
0,00031 |
0,0159 |
0,00046 |
0,023 |
0,00074 |
0,037 |
|
|
DDT |
0,0005 |
0,000207 |
0,414 |
0,00037 |
0,75 |
0,00054 |
1,08 |
0,00085 |
1,71 |
|
|
DDE |
0,0005 |
0,000093 |
0,186 |
0,00015 |
0,308 |
0,00023 |
0,47 |
0,00034 |
0,68 |
|
|
DDD |
0,0005 |
0,000045 |
0,091 |
0,0001 |
0,216 |
0,000091 |
0,18 |
0,00015 |
0,3 |
|
|
|
|
|
0,732 |
|
1,34 |
|
1,83 |
|
2,8 |
|
∑HI |
|
|
|
|
|
|
|
|
|
|
|
Lubumbashi |
||||||||||
|
|
Endosulfan |
0,0006 |
0,00094 |
0,15 |
0,0008 |
0,14 |
0,00084 |
0,141 |
0,00053 |
0,08 |
|
|
Malathion |
200 |
0,0011 |
0,055 |
0,0009 |
0,048 |
0,00096 |
0,048 |
0,00059 |
0,029 |
|
|
DDT |
0,0005 |
0,0014 |
2,8 |
0,0012 |
2,41 |
0,0012 |
2,42 |
0,00075 |
1,51 |
|
|
DDE |
0,0005 |
0,00024 |
0,48 |
0,00019 |
0,38 |
0,000192 |
0,38 |
0,00012 |
0,25 |
|
|
DDD |
0,0005 |
0,00012 |
0,256 |
0,0001 |
0,216 |
0,0001 |
0,216 |
0,00012 |
0,254 |
|
∑HI |
|
|
|
3,75 |
|
3,2 |
|
3,21 |
|
2,14 |
Carcinogenic Health Risk Assessment
Table 4 and Table 5 display the potential non-carcinogenic health risk estimations based on the HQ and HI of pesticide residues detected in vegetables for adults and children, respectively. For adult category, estimation of carcinogenic risks of pesticide residues of vegetable samples in the two cities (Kinshasa and Lubumbashi) does not reveal any risk to consumers, and this for all pesticides in each type of vegetables HR < 1 [44]. This result is consistent with earlier, Bloor et al. reported that carcinogenic risk values for vegetables from all selected farms in Ghana were < 1. In contrast, Adeoluwa ‘s research in Nigeria has rather evoked HR > 1 for aldrin and dieldrin in amaranth which could pose carcinogenic risk to consumers. Carcinogenic health risk assessment of DDTs in vegetables is a concern of public health in children group from Lubumbashi [27, 28]. The HR values in the samples of sorrel, sweet potato leaves and amaranth were 1.2, 1.05 and 1.1 respectively. Thus, there is probability of individuals (both children and adult) developing cancer over a lifetime because of exposure to DDTs residues in vegetables in DRC.
Table 4: Adult Potential carcinogenic health risk estimation of pesticide
residues in vegetables from Kinshasa and Lubumbashi markets
|
|
|
|
Sorrel |
Sweet potato-leaves |
Amaranth |
Spinach |
||||
|
|
|
CBC |
EADI |
HRI |
EADI |
HRI |
EADI |
HRI |
EADI |
HRI |
|
Kinshasa |
|
|
|
|
|
|
|
|
|
|
|
|
DDT |
0,00142 |
0,000059 |
0,041 |
0,000044 |
0,075 |
0,00015 |
0,002 |
0,000035 |
0,00046 |
|
|
DDE |
0,00142 |
0,000026 |
0,018 |
0,000044 |
0,031 |
0,000067 |
0,0021 |
0,000037 |
0,00012 |
|
|
DDD |
0,002 |
0,000013 |
0,0065 |
0,00003 |
0,015 |
0,000026 |
0,0017 |
0,000029 |
0,00019 |
|
Lubumbashi |
||||||||||
|
|
DDT |
0,00142 |
0,0004 |
0,28 |
0,00034 |
0,24 |
0,00034 |
0,0014 |
0,000024 |
0,00001 |
|
|
DDE |
0,00142 |
0,000068 |
0,049 |
0,000054 |
0,038 |
0,000054 |
0,0014 |
0,000024 |
0,000064 |
|
|
DDD |
0,002 |
0,000036 |
0,018 |
0,00003 |
0,015 |
0,00003 |
0,002 |
0,000034 |
0,0022 |
Table 5: Children Potential carcinogenic health risk estimation of pesticide
residues in vegetables from Kinshasa and Lubumbashi markets
|
|
|
|
Sorrel |
Sweet potato-leaves |
Amaranth |
Spinach |
||||
|
|
|
CBC |
EADI |
HRI |
EADI |
HRI |
EADI |
HRI |
EADI |
HRI |
|
Kinshasa |
|
|
|
|
|
|
|
|
|
|
|
|
DDT |
0,00041 |
0,000059 |
0,14 |
0,0001 |
0,26 |
0,00015 |
0,37 |
0,0065 |
0,15 |
|
|
DDE |
0,00041 |
0,000026 |
0,064 |
0,000044 |
0,1 |
0,000067 |
0,16 |
0,0028 |
6,9 |
|
|
DDD |
0,00041 |
0,000013 |
0,031 |
0,00003 |
0,07 |
0,000026 |
0,063 |
0,0011 |
2,6 |
|
Lubumbashi |
||||||||||
|
|
DDT |
0,00041 |
0,0004 |
0,97 |
0,00034 |
0,83 |
0,00034 |
0,84 |
0,014 |
0,35 |
|
|
DDE |
0,00041 |
0,000068 |
0,16 |
0,000054 |
0,13 |
0,000054 |
0,13 |
0,0023 |
5,6 |
|
|
DDD |
0,00041 |
0,000036 |
0,089 |
0,00003 |
0,075 |
0,00003 |
0,075 |
0,0013 |
3,1 |
Conclusion
This study quantified pesticide residues in vegetables commonly consumed in DRC which could potentially threaten people’s health. Risks were mainly associated with the residues of DDTs pesticides in vegetables. The HQ and HI estimations revealed a serious potential risk to consumers, children particularly. Due to multiple pesticide residues exceeding the MRLs for single residue levels, the consumers are exposed to pesticides, heavily in Lubumbashi. Due to increasing trend in pesticide use, continuous monitoring of pesticide residues in vegetables and other food is recommended in order to develop the base line data on which pesticide regulations could be enhanced in DRC.
Declarations Ethical Approval: The research protocol was approved by the Bio-ethics Committee of the School of Public Health at the University of Kinshasa. Kinshasa, DR Congo.
Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ Contributions: The first draft of this manuscript has been written by the first author P. M.N. The co-authors L. M. M. and Y.N. prepared Figures 1, 2, Tables 1, 2, 3, 4 and 5. The co- authors J. D-P. N .and J.K.T reviewed equally the manuscript. The J.K.T. contributed to supervise all the work and to correspond with the Journal.
Funding
No funding. No specific funds were received for conducting this study.
Availability of data and materials not applicable. However: the study results will report to individuals sample donors with proper explanations.
Acknowledgements: We are highly indebted to the study participants and to the staff of investigators, as well as all the local health services and health centers of the Kinshasa and Lubumbashi Public Health Systems that supported the field work. This work was received no financial support.
References
- Guler GO, Cakmak YS, Dagli Z, Aktumsek A, Ozparlak H (2010) Organochlorine pesticide residues in wheat from Konya region, Food Chem. Toxicol 48: 1218-1222.
- (2018) FAO/WHO World Food and Agriculture - Statistical Pocketbook 1-254.
- (2018) EU-2018/832. COMMISSION REGULATION (EU) 2018/ 832 - of 5 June 2018 - amending Annexes II, III and V to Regulation (EC) No 396 / 2005 of the European Parliament and of the Council as regards maximum residue levels for cyantraniliprole, cymoxanil, deltamethrin, difenoconazole, fenamidone, flubendiamide, fluopicolide, folpet, fosetyl, mandestrobin, mepiquat, metazachlor, propamocarb, propargite, pyrimethanil, sulfoxaflor and trifloxystrobin in or on certain products (europa.eu) 13: 537-544.
- Chen C, Qian Y, Liu X, Tao C, Liang Y, et al. (2012) Risk assessment of chlorpyrifos on rice and cabbage in China. Reg. Toxicol. Pharmacol 62: 125-130.
- Gad Alla SA, Loutfy N M, Shendy A H, Ahmed MT (2015) Hazard index, a tool for a long-term risk assessment of pesticide residues in some commodities, a pilot Reg. Toxicol. Pharmacol 73: 985-991.
- Giulia Bellisai, Giovanni Bernascon, Marco Binaglia, Luis Carrasco Cabrera (2023) EFSA (European Food Safety). Targeted review of maximum residue levels (MRLs) for endosulfan. European Food Safety Authority (EFSA) 21:
- Lehman E, Turrero N, Marius Kolia M, Konaté Y (2017) Felippe de Alencastro, Dietary risk assessment of pesticides from vegetables and drinking water in gardening areas in Burkina Faso. Sci. Tot. Environ 601: 1208-1216.
- ATSDR (2003) Toxicological Profile for Malathion, U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Update Statement Available online 1-19.
- ATSDR (2015) Toxicological profile for endosulfan, U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Update Statement Available online 1-20.
- ATSDR (2022) Toxicological Profile for DDT, DDE, and DDD, S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Update Statement, Available online 1-13.
- Rogan W J, Chen A (2005) Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). The Lancet 366: 763-773.
- Harada KH, Tanaka K, Sakamoto H, Imanaka M, Niisoe T, et al. (2016) Biological monitoring of human exposure to neonicotinoids using urine samples, and neonicotinoid excretion kinetics. PLoS ONE 11: e0146335.
- Yu R, Qiang Liu Q, Liu J, Wang Q, Wang Y(2016) Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun, Northeast China. Food Control 60: 353360.
- IARC (2017) International Agency for Research on Cancer - IARC Monographs on the Evaluation of Carcinogenic Risks to Humans-Volume 112: Some Organophosphate Insecticides and Herbicides. World Health Organization, Geneva. Retrieved 8: 1-2.
- Ngom S (2012) Contamination des produits agricoles et de la nappe phréatique par les pesticides dans la zone des Niayes au Sénégal, Rev. Sci. , Synthèse 5: 119-130.
- Ngweme A, Al Salah D M M, Laffite A (2020) Occurrence of organic micropollutants and human health risk assessment based on consumption of Amaranthus viridis, Kinshasa in the Democratic Republic of the Sci. Tot. Environ 1: 1-728.
- Mahmood I, Imadi S R, Shazadi K, Gul A, Hakeem K R (2016) Effects of Pesticides on Environment. Springer International Publishing Switzerland 1-18.
- Keutgen C (2013) Les maraîchers urbains de Lubumbashi : artisans de paix 11 janvier 14: 55-61.
- Matondo P N, Nuapia Y, Phanzu ND (2023) Evaluation des pratiques phytosanitaires et analyse de leurs déterminants en République Démocratique du Congo : enquête auprès de 500 maraichers des villes de Kinshasa et Lubumbashi en. Unpublished data 32: 1-5.
- Y Nuapia Y, Chimuka E, Cukrowska (2016) Assessment of organochlorine pesticide residues in raw food samples from open markets in two African cities, Chemosphere 164: 480-487.
- Cook S, M Khan, Z R Pickett JA (2007) The use of push-pull strategies in integrated pest An. Rev. Entomol 52: 375-400.
- Dorothea F K, Roscoe R J (2010) Application of the QuEChERS method for the analysis of pyrethrin and pyrethroids in fish tissues. Analytical Bioanalytical Chemistry 397: 2525-2531.
- Olatunde S, Olatunji (2019) Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MSSCieNtifiC REPOrTS 9: 1-538
- FAO, FAOSTAT, Rome: FAO, 2020. http://www.fao.org/faostat/en/#home.
- PMFAI-Pest www.pmfai.org/stat.htm
- Akoto H Ant (2013) Health risk assessment of pesticides residue in maize and cowpea from Ejura, , Chemosphere 92: 67-73.
- Oyekunle J A, Akindolani OA, Sosan M B, Adekunle A S (2017) Organochlorine pesticide residues in dried cocoa beans obtained from cocoa stores at Ondo and Ile-Ife, Southwestern Nigeria, National library of medicine toxrep Collection 6: 151-159.
- Dougherty C P, Holtz S H, Reinert JC, Panyacosit L, Axelrad T, et (2000) Dietary exposures to food contaminants across the United States. Environ. Res 84: 170-185.
- Forkuoh N, Owusu L, Sheringham S (2018) Risk of Human Dietary Exposure to Organochlorine Pesticide Residues in Fruits from Ghana Frederick. Scientific Report 8: 16686.
- Wang HS, Jamal J D (2011) Daily intake, and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data 192: 1441-1449.
- Aamir M, Khan S, Li G (2018) Dietary exposure to HCH and DDT congeners and their associated cancer risk based on Pakistani food consumption. Environ 25: 8465-8474.
- (2014) US-EPA United State Environmental Agency, Plan EJ 2014, Risk assessment Guidance, USEPA Risk Assessment Guidance | US EPA. Reviewed 9-11.
- Kolani L, Mawussi G, Sanda K (2016) Assessment of Organochlorine Pesticide Residues in Vegetable Samples from Some Agricultural Areas in Am. J. Anal. Chem 7: 332-341.
- Lehmann E, Turrero N, Kolia M, Konaté Y (2017) Felippe De Alencastro, Dietary risk assessment of pesticides from vegetables and drinking water in gardening areas in Burkina Faso. Sci. Tot. Environ 601: 1208-1216.
- Jallow M F A, Awadh D G, Albaho M S, Devi V Y, Ahmad N (2017) Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Int. J. Environ. Res. Public Health 14: 833.
- Kariathi V, Kassim N, Kimanya M (2016) Pesticide exposure from fresh tomatoes and its relationship with pesticide application practices in Meru district. Cogent. Food Agric 2: 1196808.
- Diop A, DiopYM, Thiaré D D, Cazier F, Sarr S O, et (2016) Monitoring survey of the use patterns and pesticide residues on vegetables in the Niayes zone, Senegal. Chemosphere 144: 1715-1721.
- Erhunmwunse NO, Dirisu A, Olomukoro JO (2012) Implications of pesticide usage in Nigeria, Freshwater Biol 21: 15-25.
- Amoah P, Drechsler P, Abaidoo R C, Ntow W J (2006) Archive Pesticide and pathogen contamination of vegetables in Ghana’s urban Environ. Contam. Toxicol 50: 1-6.
- Latif Y, Sherazi STH, Bhanger M I (2011) Assessment of pesticide residues in commonly used vegetables in Hyderabad, Ecotoxicol. Environ. Safety 74: 2299-2303.
- Oluwaseyi A, Adeleye A, Sosan M B, Oyekunle, JAO (2005) Dietary exposure assessment of organochlorine pesticides in two commonly grown leafy vegetables in South-western, Nigeria Department of Crop Production and Protection, Obafemi Awolowo University, Obafemi Awolowo University, Nigeria Ile-Ife 24: 165-184.
- Nisha U S, Khan SI, Hossain M D (2021) Quantification of Pesticide Residues in Fresh Vegetables Available in Local Markets for Human Consumption and the Associated Health Agronomy 11: 1-11.
- Labie D (2010) Le’s relations complexes entre hémoglobinopathies et paludisme, The complex relations between hémoglobinopathies and malaria, Sci 26: 685- 687.
- Bolor VK, Boadi N O, Borquaye L S, Afful S (2018) Human Risk Assessment of Organochlorine Pesticide Residues in Vegetables from Kumasi, Ghana, Hindawi J. Chem 1-11.

