Antimicrobial Activity of Acacia Nilotica Pods Against Carbapenem Resistant Gram-Negative Bacteria Isolated from Different Hospitals in Khartoum State
© 2021 Umkalthoum Mohemed Taher Mohemed Alamein, Babbiker Mohammed Taher Gorish, kawthar Abdelgaleil Mohammed Salih, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This study was aimed to investigate the antimicrobial activity of ethanolic extract of Acacia nilotica against carbapenem resistant bacteria during the period from February 2018 to December 2018. Agar disc diffusion method was used to determine the antimicrobial activity of Acacia nilotica and the ethanolic extract was examined against carbapenem resistant Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter cloacae and Citrobacter freundii isolated from different hospital in Khartoum state and the sample include (urine, wound swab, sputum, blood and body fluid) for 91 clinical isolates. The extract show activity against all tested microorganism and the inhibition zone were17±3 mm for E. coli, 18.3±4mm for Klebsiella pneumoniae, 16.9±4mm for Proteus mirabilis, 17±3 mm for Pseudomonas aeruginosa, 18.3±0.5mm for Enterobacter cloacae and 16±5mm for Citrobacter freundii and the activity of the extract at 100 mg/ml concentration show sensitive (82.4%), (72.6%) for 50mg/ml and (51.6%) for 25 mg/ml for all tested bacteria. Ethanol extract of Acacia nilotica was found to be effective as antibacterial against different bacterial pathogens, providing the scientific basis for its traditional application in Sudanese folk medicine against many bacterial diseases, extract had an in vitro antibacterial activity against carbapenem resistant bacteria, further studies are required to confirm this result to identify active ingredient and toxicity.
Introduction
Since 1970, resistance to antimicrobial has become an escalating problem [1]. Effective antimicrobial strategies developing drive from the fact that the bacteria were uniquely suited for survival in toxic environment, the ability of bacteria to subsist on antibiotics and the potential to acquire resistance gene as a growing concern [2].
Plants can be an effective source of antimicrobials and had been used for centuries traditionally to inhibit microbial growth [3]. Acacia nilotica contains secondary metabolites including amines and alkaloids such as cyanogenic glycosides, cyclitols, fatty acids and seed oils, fluoroacetate, gums, nonprotein amino acids, terpenes, flavonoids and condensed tannins [3]. The emerging phenomenon of multidrug resistant (MDR) Gram-negative bacilli (GNB) is a pressing contemporary concern. This challenge has been compounded by the paucity of new antibiotics in late-stage development for MDR- GNB [4].
The carbapenems are considered the last resort antibiotics used for infections caused by MDR-GNB due to their stability against beta-lactamases, penicillinases and cephalosporinases, and their broad spectrum of action [5]. The global spread of carbapenemase- producing Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter species (i.e., multidrug-resistant organisms [MDROs] is a critical medical and public health issue [6]. These bacteria are often resistant to all beta-lactam agents and are frequently co-resistant to multiple classes of other antimicrobial agents, leaving very few treatment options [6].
The problem of microbial resistance is growing and the outlook for use of antimicrobial drugs in the future is still uncertain, therefore, action must be taken to reduce this problem, for example, to control the use of antibiotic, develop research to better understand the genetic mechanism of resistance, and to continue studies to develop new drugs, either synthetic or natural [6]. Furthermore, antibiotic resistance is a global challenge that impacts all pharmaceutically used antibiotic, in recent years pharmaceutical companies have almost stopped producing new antibiotics which have led researchers to look for alternative antimicrobial, Herbs were used for the treatment of infectious diseases in many developing countries [7]. Plant possesses active ingredient for defense against plant pathogen many of these antimicrobial substances were found to produce the same effect against human pathogen, there for the researchers started to screening plenty of plant extract and essential oil against various human pathogen, screening medical plant for antimicrobial sensitivity has led to encouraging result however it is essential to investigate the toxicity of plant as well [7]. Acacia nilotica possesses antibacterial activity because it used in rural medical care for treatment of many infectious and chronic diseases, thus, to verify the antibacterial activity of those plant against resistant bacteria isolated from deferent clinical sample.
In Sudan, with high percentage of multidrug resistant bacteria, we in urgent need to develop new drug from our traditional medicine. Therefore, our study was attempt to solve problem of resistant, found good treatment for bacterial disease.
Materials and Methods
This study was a descriptive cross-sectional study, During the period from February to December 2018 a total of ninety- one clinical isolates were obtained from clinical specimens of Hospitalized patients in different hospitals in Khartoum state including Military hospital, Omdurman Teaching Hospital, Fedial hospital, Ultra laboratory, Alacadimy Hospital and Police Education Hospital. All Gram negative carbapenem resistant bacteria were Included in this study, all isolates that are ither non-Gram negative or carbapenem susceptible are excluded from this study.
Data Collection
Data were collected from the isolates using data collection form containing all study variables
Ethical Consideration
Permission was issued by the Medical Laboratory Science Collage’s Ethical Committee; Sudan University of Science and Technology the consent was taken from Laboratory Manager to collect specimens with ensuring all ethical consideration for conducting the research in a way that protect the patient’s privacy
Collection Of plant Samples
The plant extracts were collected and authenticated at the Medicinal and Aromatic plant Research Institute (MAPRI).
Preparation of the Extract
Extraction was carried out according to method descried by Sukhdev et.al, Hundred gram of the plant samples were grounded using mortar and pestle and extracted by soaking in 80 % ethanol for about five days with daily filtration and evaporation. Solvent was evaporated under reduced pressure to dryness using rotary evaporator apparatus and the extract allowed to air till complete dryness and the yield percentages were calculated [8].
Collection of samples
Various clinical samples including (wound swab, urine, blood and sputum) were collected, inoculated in basic media and selective media then identified and preserved in glycerol peptone water, the initial collection and processing of the specimen was done in hospital then further investigation and sensitivity was done at Research Laboratory in Sudan University for Science and Technology.
Identification
Biochemical test for Gram’s negative rods were carried out according by Chesbrough [9].
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing of Gram-Negative Bacilli isolates will be performed on Muller-Hinton Agar (MHA) plate (Oxoid, UK) by the KirbyBauer disk diffusion method following the Clinical and Laboratory Standards Institute guidelines [10]. The antimicrobial agents which were tested from different categories including: extended spectrum cephalosporins [ceftazidime (30 μg), antipseudomonal penicillin’s with βlactamase inhibitors carbapenems [imipenem (10μg), meropenem (10 μg)], aminoglycosides [gentamicin (10 μg), amikacin (30 μg)], fluoroquinolones [ciprofloxacin (5 μg), folate pathway inhibitor [co trimoxazole (25 μg)], and polymyxin [colistin (10 μg)] (Oxoid, UK). E. coli ATCC 25922 were used as control strains and tested each time when susceptibility testing was performed, zone diameters of each of the antibiotics will be interpreted as per CLSI recommendations (10).
Sensitivity Testing
Muller Hinton media susceptibility of common and rapid growing bacteria using antimicrobial by Kirby – Bauer method, antimicrobial to be included in sensitivity test well depend on pathogen and rang of locally available antimicrobial [9].
Preparation of Bacterial Suspension
The inoculum density was compared with McFarland standard solution of BaSO4 (0.1ml of 1% BaCl2 + 9.9ml of 1% H2SO4). The suspension was stored in the refrigerator at 4ºC until used.
Modified Kirby Bauer Method
Three to five colonies of similar appearance were touch and emulsified in 3to 4 ml of normal saline or nutrient broth , in good light the turbidity of the suspension were matched with turbidity of McFarland stander against piece of paper Muller Hinton agar was seed by using sterile cotton swab and the surface of the media allow to dry , then by sterile forceps apply the disc about 15mm from the edge and 25mm, then incubate the plate in incubator at 37C0 for 18-24 hour interpretation of zone size by interpretative chart either to be sensitive ,intermediate and resistant [9].
Determination of Ethanolic Extract of Acacia nilotica Activity By Disc diffusion method with some modifications; In aseptic conditions, 20 ml of warm Mueller Hinton agar (Watin-Biolife, KSA), was poured on sterile disposable plates (Jalil Medicals) and left at room temperature to solidify, then turned upside down and kept in the refrigerator for about halve hour. 100 μl of the bacterial suspensions (previously adjusted) were swapped onto the Mueller Hinton plates, using sterile cotton swaps. Sterile blank discs of 6 mm were previously prepared from Whatman No.1 filter paper (Sigma-Aldrich) and saturated with 100,50 and 25 mg/ml to trap about 8 and 4 mg/disc, respectively (Pre-experimental measurements showed that the 6 mm disc absorb about 20 μl). Saturated discs were placed onto inoculated plates, the plates were allowed to stand for a while at room temperature, and then incubated at 37°C for 24 hrs. The susceptibility of the tested bacteria to the extract was indicated after incubation by zones of growth inhibition in millimeter (mm) using a transparent ruler. Gentamicin discs (10 μg/disc) (Oxoid), were used as standard antibacterial (positive control), other discs saturated with the solvent (ethanol) were loaded in a separate inoculated plate and served as negative control [11].
Phenotypic Detection of Carbapenemase
All isolates resistant to meropenem and imipenem in Kirby-Bauer disk diffusion method would be confirmed for carbapenemase production by Modified Hodge Test (MHT). In the test, inoculum of E. coli ATCC 25922 (comparable to 0.5 McFarland standard), would be inoculated on MHA. Two discs of meropenem and imipenem (10 μg) would be placed on the surface of MHA 30 mm opposite to each other in a straight line, the test organisms will be streaked from the edge of one disk meropenem to edge of the other imipenem disk. The plates would be incubated at 37 °C for 24 h. They would be examined for a clover leaf type indentation or flattening at the intersection of the test organism and E. coli ATCC 25922 within the zone of inhibition of the carbapenem susceptibility disc as described by [12].
Phenotypic Detection of Carbapenemase Producer for MBL Isolates that will be found resistant to imipenem, meropenem or third generation cephalosporins (ceftazidime) in Kirby Bauer disk diffusion method will presumptively considered MBL producers and will be confirmed by the Imipenem-EDTA Combined Disc Test (CDT). Briefly, the test inoculums (comparable to 0.5 McFarland standards) will be inoculated MHA plates. Two imipenem (10ug) disks will be placed on the surface of agar plate and 10 μl EDTA solutions will be added to one of them to obtain a desired concentration of 750 μg. The plates will be incubated for 24 hours at 35°C. An increase in zone size of ≥7 mm for imipenem-EDTA disk compared to imipenem disk alone will indicate MBL producer strain as described by [13].
Data Analysis
The Data analyzes was carried out through statistical package for the social science (SPSS) version 20 (one sample T-test and other statistical method e.g., mean and stander deviassion percentage and frequency), and excel programs.
Results
The bacteria are isolated and identified were 91 (31.9%) and were carbapenem resistant bacteria, E. coli were most abundant 40 (44%), followed by Klebsiella pneumoniae 25(27.8%) followed by Proteus mirabilis were 14 (15.6), Pseudomonas aeruginosa were 5 (5.5), Enterobacter cloacae were 3 (3.3 %) and Citrobacter freundii were 3 (3.3%) table (1). Most predominant sample type contain carbapenem resistant bacteria was wound swab 36 (40%) then followed by urine sample 35 (38.9%), sputum sample 15 (16.7), fluid sample (2.2 %) and blood sample represent 2 (2.2 %). Table (2). The result of antimicrobial agents showed that most effective antibiotic against carbapenem resistant isolated bacteria was colistin 75 /91(83.3 %), followed by Amikacin 40/91 (54.4%), cotrimoxazol 18/91 (20%) and more resistant antibiotic was ceftazidime 86 /91 (95. %) followed by ciprofloxacin 84/91 (92.3%) table (3). Modified hodge test (Figure 2) show 57/91(62.7%) was positive and 34/91 (37,3 %) was negative and Imipenem- EDTA Combined Disc Test (figure3) show 63/91 (69.2%) was positive and 28/91 ( 30.8%) was negative . the extract activity (Figure1-4) against all tested microorganisms and the inhibition zone were E.coli 17±3 mm, Klebsiella pneumoniae 18.3±4,mm Proteus mirabilis 16.9±4mm, Pseudomonas aeruginosa 17±3 mm , Enterobacter cloacae 18.3±0.5mm, Citrobacter freundii 16±5mm and the activity of the extract at 100 mg/ml concentration show Sensitive 75/91 (82.4%) and resist 15(17.6%), 66/91 (72.6%) for 50mg/ml resistant represent 25/91 (27.4%) and 47/91 (51.6%) for 25 mg/ml and the resistant was 44/91 ( 48.3%) for all tested bacteria (table 4). Antimicrobial activity of Acacia nilotica against all bacterial type are presented in Table (5) Effectivity of Acacia nilotica verses drugs(antibiotic) are presented in Table (6)
Table 1: Percentage and frequency of isolated bacteria
|
Bacterial isolate |
Frequency |
Percentage % |
|
E. coli |
40 |
44% |
|
Klebsiella pneumoniae |
25 |
27.8% |
|
Proteus mirabilis |
14 |
15.6% |
|
Pseudomonas aeruginosa |
5 |
5.5% |
|
Enterobacter cloacae |
3 |
3.3% |
|
Citrobacter freundii |
3 |
3.3% |
|
Total |
91 |
100% |
Table 2: Frequency and percentage of clinical sample
|
Sample |
Frequency |
Percentage |
|
Blood |
2 |
2.2% |
|
Fluid |
2 |
2.2% |
|
Sputum |
15 |
16.7% |
|
Urine |
35 |
38.9% |
|
Wound swab |
36 |
40 % |
Table 3: Percentage and frequency of drug sensitivity
|
Antibiotic |
Sensitive |
Resistant |
|
Ceftazidime |
4 / 91 (4.4%) |
87/91 (95.6%) |
|
Cotrimoxazole |
18/91 (19.8%) |
73/91 (80.2%) |
|
Ciprofloxacin |
7/91 (7.7%) |
84/91 (92.3%) |
|
Imipenem |
0/91 (0%) |
91/91 (100%) |
|
Amikacin |
40/91 (54.0 %) |
51/91 (56%) |
|
Colistin |
75/91 (82.4%) |
16/91 (17.6%) |
Table 4: Antimicrobial activity of Acacia nilotica against isolated bacteria
|
AN concentration |
Sensitive |
Resistant |
p.value |
|
100% |
75/91 (82.4%) |
16/91 (17.6%) |
0.0 |
|
50% |
66/91 (72.6%) |
25/91 (27.4%) |
0.491 |
|
25% |
47/91 (51.6%) |
44/91 (48.3%) |
0.006 |
Key: (AN: Acacia nilotica, SD: stander deviation)
Table 5: Bacterial isolate and plant concentration (sensitive and Resistant)
|
Isolates |
Sensitive/ Concentration |
Resistant/ Concentration |
|||||||
|
|
100% |
p. value |
50% |
p. value |
25% |
p. value |
100% |
50% |
25% |
|
E. coli |
33/40 (82.5%) |
0.001 |
26/40 (65%) |
0.363 |
21/40 (52.5%) |
0.047 |
7/40 (11.5%) |
14/40 (35%) |
19/40 (47.5%) |
|
Klebsiella pneumoniae |
25/25 (100%) |
0.001 |
21/25 (84%) |
0.027 |
17/25 (68%) |
0.410 |
0/25 (0%) |
4/25 (16%) |
8/25 (32%) |
|
Proteus mirabilis |
13/14 (92.9%) |
0.104 |
10/14 (71.4%) |
0.197 |
4/14 (28.6%) |
0.002 |
1/14 (7.1%) |
4/14 (28.6%) |
10/14 (71.4%) |
|
Citrobacter freundii |
2/3 (66.7%) |
0.775 |
1/3 (33.3%) |
0.504 |
1/3 (33.3%) |
0.390 |
1/3 (33.3%) |
2/3 (66.7%) |
2/3 (66.7%) |
|
Enterobacter cloacae |
3/3 (100%) |
0.010 |
3/3 (100%) |
0.423 |
1/3 (33.3%) |
0.122 |
0/3 (0%) |
0/3 (0%) |
2/3 (66.7%) |
|
Pseudomonas aeruginosa |
5/5 (100%) |
.075 |
4/5 (80%) |
1.000 |
2/5 (40%) |
0.463 |
0/5 (0%) |
1/5 (20%) |
3/5 (60%) |
Table 6: Effectivity of Acacia Nilotica Against Drugs (Antibiotics)
|
Isolates |
Acacia Nilotica |
Ceftazidime |
Ciprofloxacin |
Cotrimoxazole |
imipenem |
Amikacin |
Colistin |
||
|
|
100% |
50% |
25% |
|
|
|
|
|
|
|
E. coli |
33/40 (82.5%) |
26/40 (65%) |
21/40 (52.5%) |
2/40 (5%) |
2/40 (5%) |
8/40 (20%) |
0/40 (0%) |
18/40 (45%) |
40/40 (100%) |
|
Klebsiella pneumoniae |
25/25 (100%) |
21/25 (84%) |
17/25 (68%) |
0/25 (0%) |
2/25 (8%) |
4/25 (16%) |
0/40 (0%) |
14/25 (56%) |
22/25 (88%) |
|
Proteus mirabilis |
13/14 (92.9%) |
10/14 (71.4%) |
4/14 (28.6%) |
2/14 (14.3%) |
1/14 (7.1%) |
4/14 (28.5%) |
0/40 (0%) |
5/14 (35.7%) |
7/14 (50%) |
|
Pseudomonad as aeruginosa |
5/5 (100%) |
4/5 (80%) |
2/5 (40%) |
0/5 (0%) |
0/5 (0%) |
0/5 (0%) |
0/40 (0%) |
2/5 (40%) |
2/5 (40%) |
|
Enterobacterr cloacae |
3/3 (100%) |
3/3 (100%) |
1/3 (33.3%) |
0/3 (0%) |
2/3 (66.7%) |
2/3 (66.7%) |
0/40 (0%) |
1/3 (33.3%) |
2/3 (66.7%) |
|
Citrobacter freundii |
2/3 (66.7%) |
1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0%) |
0/3 (0%) |
0/3 (0%) |
0/40 (0%) |
0/3 (0%) |
2/3 (66.7%) |

Figure 1: Acacia nilotica pods
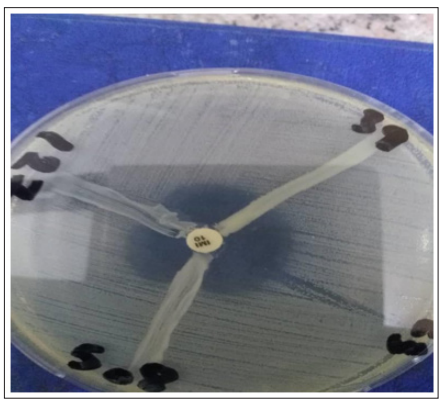
Figure 2: The Modified hodge test (MHT) performed on a Muller Hinton Agar plate. (508,127) MHT positive result (39) MHT negative result.

Figure 3: Positive imipenem-EDTA combined disc test for one of the isolated Gram-negative bacteria. The imipenem + EDTA discs shows larger zone of inhibition than imipenem disc alone.
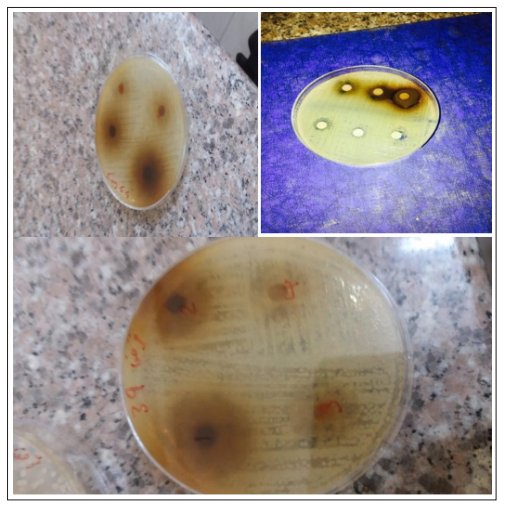
Figure 4: Acacia nilotica antimicrobial activity
Discussion
Antimicrobial drugs provide the main basis for treatment of various microbial infections; however, the high genetic variability of some microorganisms enable them to rapidly develop antimicrobial resistance. Thus, there has been a continuing search for new potent antimicrobials [14].The results of this study indicate that the ethanol extract of Acacia nilotica pods contains active compounds capable of killing a range of bacteria types, including E coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter cloacae and Citrobacter ferundii which are carbapenem resistant bacteria. And the activity effective at the highest concentration of plant extract and lower at the lower concentration for all tested bacteria that agreement with study although it use differ bacterial species reported that Escherichia coli, followed by Staphylococcus aureus , Acinetobacter baumanni, and Bacillus cereus ATCC 11778 bactericidal effect at high concentrations and bacteriostatic at lower concentrations, and other study also agreement with my study found and the most effective against E.coli. But my study done on carbapenem resistant bacteria show that all the test organisms were found to be sensitive to all the extracts prepared in the study. Maximum activity with respect to zone of inhibition was recorded for E. coli followed by S. pyogenes, B. cereus, V. cholerae, S. aureus, B subtilis, Aeruginosa, S. dysenteriae, C. perfringens, S. typhi, Lmonocytogenes, while C [11].
Jejuni was found to be least sensitive with no activity recorded with acetone extract (P<0.001. and also, study done on multi drug resistant [15]. Also, agreement with study (16) but differ in the solvent used and the bacterial type show the antibacterial activity of aqueous extract against Staphylococcus aureus and Pseudomonas aeruginosa. Standard drug used as control recorded zone of inhibition on the test bacteria, Chloroform extract was found to be the least effective as compared to aqueous extract with diameter zone of inhibition and compared the finding against S aureus and P. aeruginosa this study use different type of the extract but it show the activity of Acacia nilotica against the bacterial isolate even use other type of extract also The activity of the standard control was more effective, also agreement with Acacia nilotica leaves and park extraction against S. aureus, E.coli, P.aeruginosa, P. mirablis, Acinetobacter spp and Candida albicans in the acetonic leave extract the zone of inhibition of S. aureus followed by P. mirablis E.coli and Acinetobacter spp, Candida albicans and Pseudomonas spp, this study done only on park not in leaves therefore get differ in the result but also give activity against bacterial isolate .
My study disagreement with. In the activity concentration (lower more active than highest one) and in the type of control antibiotic used the lowest concentration (10%) of the extract, showed a high antibacterial activity against Staphylococcus aureus and a low antibacterial activity against Klebsiella pneumoniae highest activity was due to the action of ciprofloxacin and gentamycin against Pseudomonas aeruginosa and K. pneumoniae. While the lowest activity was due to the action of amoxicillin against Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa There was an insignificant difference (p > 0.05) [16- 18].
The present study was done on carbapenem resistant other study done on ESβL the lysates showed remarkable bactericidal properties, killing almost 100 % of the bacteria they were tested against, including neuropathogenic Escherichia coli, MRSA, and Klebsiella spp. The bactericidal activity was heat-resistant and showed minimal cytotoxic effects on human brain microvascular endothelial cells. FPLC revealed eight peaks, with three of them representing compounds that had maximum bactericidal activity against all the tested isolates, but showed < 30 % host cell cytotoxicity determination of cytotoxicity ranged from 8.1 – 29.0 %, depending on the fraction [19]. And it not detected in my research.
The differences may be attributed to the fact that effectiveness of the extracts largely depends on the kind of solvent used. Further, the concentration of the extract and kind of bacteria may also account for the difference in the susceptibility pattern of the test organism. There were few researches about effect of Acacia Nilotic a on carbapenem resistant bacteria. Therefore, this study determined the value of Acacia nilotica plant as alternative treatment for bacterial infections and carbapenem resistant bacteria that can be used to completely overcome or minimize the resistance of bacteria observed in synthetic antimicrobial agents.
Conclusion
Ethanolic extract of Acacia nilotica was found to be effective as antibacterial against different bacterial pathogens, providing the scientific basis for its traditional application in Sudanese folk medicine against many bacterial diseases. Good activity of Ethanol extract of Acacia nilotica at highest concentration (100 mg/ml), Poor activity at lower concentration at 25mg /ml. Acacia nilotica extract had an in vitro antibacterial activity against carbapenem resistant bacteria which are E. coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae, Proteus mirabilis, Citrobacter ferundii and Enterobacter cloacae. It may be used as an alternative treatment of infections associated with these organisms.
Recommendation
Further investigation into pharmacological properties of secondary metabolites of Acacia nilotica, more research is required to understand the mode of actions of these plants. Further studies should be carried out for the isolation and characterization of the bioactive compounds. Determination of minimum inhibition concentration (MICS) and toxicity for the active ingredients of each bacteria including in this study. More studies about carbapenem resistance bacteria, and can be supported by molecular detection of resistant genes.
References
- Aksoy DY and Unal S (2008) new antimicrobial agents for the treatment of Gram-positive bacterial infections; Clin Microbiol Infect 14: 411-420.
- Dantas G, Sommer MOA, Oluwasegun RD, Church GM 2008 Bacteria subsisting on antibiotics Science 320: 100-103.
- Simões M, Bennett RN, Rosa EA (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms Nat Prod Rep 26: 746–757
- Harris P, Paterson D and Rogers B (2015) Facing the challenge of multidrug-resistant Gram-negative bacilli in Australia; Med J A 202: 243-247.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA (2011) Carbapenems: past, present, and future Agents Chemother 55: 4943-4960.
- Kallen AJ and Srinivasan A (2010) Current epidemiology of multidrug resistant Gram- negative bacilli in the United States Infect Control Hosp Epidemiol 31: 551-554.
- Kavitha PA, Kumar P, Murthy TPN, Gopinath SM (2013) Methanolic extract of Acacia nilotica and antibacterial activity against Hospital isolates of Bengaluru ; Inter. J. Latest Res. Sci. Technol 2: 522-524.
- Sukhdev SH, Suman P S K, Gennaro L and Dev, D R (2008) Extraction technologies for medicinal and aromatic plants. United Nation Industrial Development Organization and the International Center for Science and High Technology
- Cheesbrough M. (2005) District Laboratory Practice in tropical countries, 2 edition part 2 8.5.3-8.5.7.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility testing; twenty first informational supplements,2011; M100-S21. 1. Vol. 31. CLSI, Wayne, Pa, USA.
- Abdallah E M (2016) Antibacterial efficacy of Acacia nilotica pods, growing in Sudan against some bacterial pathogens; Int J Curr Res Biosci Plant Biol 3: 6-11.
- Anderson, KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B and McDougal Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 45: 2723-2725.
- Yong D, Lee K, Yum JH, Shin HB, Rossolini GM and Chong Y (2002) Imipenem-EDTA disc method for differentiation of metallo-ß- lactamase-producing clinical isolates of Pseudomonas and Acinetobacter spp. J Clin Microbiol 40:3798-3801.
- Abd-Ulgadir K S, El-Rofaei N A, and El-Kamali H H (2015) Department of Microbiology –Faculty of Nursing Science, Omdurman Islamic University (O I U), P.O. Box 382, Omdurman-Sudan 4: 34-45.
- Rubina Lawrence, Ebenezer Jeyakumar and Akshi (2015) Gupta Department of Microbiology and Fermentation Technology, Sam Higginbottom Institute of Agriculture, Technology and Sciences (Deemed- to-be-University), Allahabad, Uttar Pradesh, 211007, India Int.J.Curr.Microbiol. Sci.
- Manga S S Isah, M and Danlami, M B (2018) Kebbi State University of Science and Technology, Aliero, Nigeria. Department of Biological Sciences Federal University Birnin Kebbi (Department of Microbiology) UJMR, Volume 3 ISSN: 2616-0668.
- Sharma C, Aneja R K, Dhinan R, Jiloha P, Meashi V, and Kaur M (2014) invitro evaluation of antimicrobial spectrum of acacia nilotica leaves and bark extract against pathogen causing otitis infection Journal of innovative 1:51-56
- (2017) Al Neelain University, Khartoum, Sudan, African Journal of Medical SciencesVol 2.
- Saba R, Muhammad F, Shahida H and Naveed A (2011) Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan. Tropical Journal of Pharmaceutical Research. 10: 785-879.

