Antihyperlipidemic Activity of Phormidium Tenue on High Cholesterol Diet Induced Hyperlipidemia in Wistar Albino Rats
© 2023 Ganesan Silambarasan, Thirugnanasambandam Ramanathan, Kandasamy Kathiresan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This study investigated antihyperlipidemic activity of Phormidium tenue on high cholesterol diet induced hyperlipidemia in Wistar albino rats. The ethanolic extract of P. tenue was given at the dose of 1 g/kg to the group of animals once in a day along with the high cholesterol diet orally, for 25 days. There was a significant increase in body weight of high cholesterol diet rats, compared to normal rats. However, there was no change in the body weight of test groups treated with P. tenue. The treatment also significantly reduced the levels of serum cholesterol, Triglyceride (TG), Low Density Lipoprotein (LDL) and Very Low-Density Lipoprotein (VLDL) and increased the level of High-Density Lipoprotein (HDL) in hyperlipidemic rats nearer to standard drug. Histopathological studies revealed that the coronary artery wall appeared normal, with no foam cells, no disturbance in the endothelial cell and no change in the muscle fibers in the control group or standard drug treated group. The coronary artery wall of the algal treated rats was similar to that of the normal rats. Our study suggests that P. tenue has the potential protective role for coronary heart disease, and hence further clinical trials are required to explore natural biomolecules towards developing drug.
Introduction
Elevated levels of serum cholesterol leads to atherosclerosis [1]. Hyperlipidemia is a major cause of atherosclerosis and other associated conditions, such as coronary heart disease, ischemic cerebrovascular disease, and peripheral vascular disease [2]. Among these, hypercholesterolemia and hypertriglyceridemia are closely related to ischemic heart disease [3]. Atherosclerosis is the most important pathological process leading to cardio- and cerebro-vascular diseases, is suggested to be mediated by increase in the serum lipid, thrombosis, and injuries of the endothelial cells [4]. The risk of cardiovascular diseases is multi-factorial in causation due to both modifiable and non-modifiable risk factors. The World Health Report of 2002 has listed six non- communicable disease (NCD)-related risk factors, amongst the 10 most important risk factors accounting for a large proportion of the global burden of chronic disease. The Indians have developed myocardial infarction at younger age, which is largely due to a higher prevalence of these risk factors [5]. Hyperlipidemia is the most prevalent indicator for susceptibility to atherosclerotic heart disease. It is characterized by abnormally elevated levels of lipid (triglyceride and cholesterol) and lipoprotein in the blood.
The arteriosclerosis index has also improved with no adverse effects, which is similar to the observations made by Japanese researchers [6]. West Germany researchers have discovered the cholesterol reduction during a weight loss study with Spirulina. In this study against a placebo, about 6 tablets three times a day over four weeks have showed a small but significant reduction of body weight. There is also a significant drop in serum cholesterol levels [7]. However, lower cholesterol has been observed without weight loss, suggesting that cholesterol reduction is not related to weight loss [8]. In a clinical study, a significant reduction in the ratio of Low-density lipoproteins (LDL) and high-density lipoproteins (HDL) in 15 diabetic patients who are given Spirulina. However, better studies are needed before Spirulina can be recommended [9].
Major limitations in the effective pharmacological treatment of hyperlipidemia are the constraints imposed on health care resources, particularly in the low-and middle-income countries [10]. There is a need to tackle this physiological problem as attaining grave proportions globally. In this scenario, the problem may be tackled by use of natural agents due to their cost effectiveness and minimal side-effects [11]. So far there are few potential bioactive compounds of terrestrial origin. Recently, the trend has been switched on to the oceans as a potential source for the drugs. So, the present study investigates anti-hyperlipidemic activity of marine cyanobacteria species, Phormidium tenue on high cholesterol diet induced hyperlipidemia in Wistar albino rats.
Materials and Methods
The marine cyanobacterium Phormidium tenue was collected from rhizosphere soil samples of mangroves, Parangipettai, south east coast of India. The cyanobacterium was isolated under the aseptic condition using the marine nutrient medium designed and modified by [12,13]. The species was identified with the help of classical manuals [14,15]. It was sub-cultured in conical flasks under the laboratory condition. Cyanobacterial culture at stationary growth phase was harvested by centrifugation. The culture was purified and used for various experimental purposes.
Identification
Phormidium tenue (Menegh.) Gomont
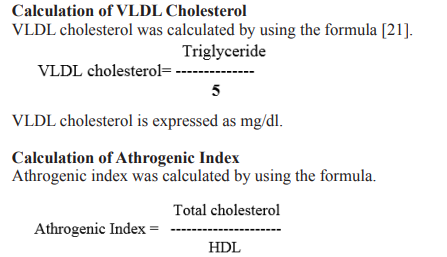
Molecular identification Gene Bank Accession Number: JQ258938 Morphological identification key characters (Fig 1): Colour of Thallus - blue green; Mass forming thin membranous layers; Filaments - very narrow, densely entangled; Sheaths very thin and delicate, somewhat inconspicuous, finally diffluent; Trichome 1-2 μ m in diameter, straight or somewhat curved, apex more or less tapering, slightly constricted at cross walls; Cells 2-3 μ m broad, 2-4 μ m long, distinctly separated by means of pellucid dessipiments; Apical cell somewhat conical or blunt; Cell contents - homogenous and pale blue-green.
Experimental Animal
Wistar albino rats (weighing 150-200 g) were housed individually in a well-ventilated room with temperature of 25 ± 2°C, humidity of 65-70% and 10 h light/dark cycle at Central Animal House, Rajah Muthiah Medical College, Annamalai University and water was provided at libitum. After adaptation, the rats were divided into four groups. All animals were fed daily with 100 g of feed. All studies were carried out in accordance with international law of animal care and use, and Institutional Animal Ethical Committee (IAEC) of Rajah Muthiah Medical College and hospital (Reg No./160/1999/CPCSEA, Ethical Approval Number: 842), Annamalai University, Annamalai Nagar, Tamil Nadu, India.
Chemical and Drug
Cholesterol (50 gm) and Sodium cholate (25 gm) were purchased from Sisco Research Laboratory Mumbai, India. Standard drug Fenofibrate was obtained from Morals labs, Chennai. Other chemicals, reagent kit for cholesterol, High density lipoproteins (HDL) cholesterol and solvent were purchased from S.D fine chemical Mumbai, India.
Induction of Hyperlipidemia
High cholesterol diet was prepared by mixing cholesterol 2%, sodium cholate 1% and coconut oil 2%, with standard powdered animal food. The diets were placed in the cage carefully and were administered for 25 days.
Test Sample
The ethanolic extract of Phormidium tenue was given at a dose of 1.0 mg/kg to the group of animals once in a day along with the high cholesterol diet orally. Treatment was given daily for 25 days.
Protocol for Experimental Studies
The experimental animals were divided into four groups for each group of six animals
Group-1: Normal rats fed with standard diet (Control) Group-2: Hyperlipidemic rats (Hyperlipidemic control)
Group-3: Hyperlipedemic rats treated with Phormidium tenue
(1.0 g/kg b.wt) orally.
Group-4: Hyperlipedemic rats treated with standard drug fenofibrate 65mg/kg.
The animals were divided in to four groups for each group with six rats. The first group of animals received normal diet only. Second group of animals received high cholesterol diet and served as hyperlipidemic control. Third group of animals received high cholesterol diet treated with Phormidium tenue (1.0 mg/kg b.wt orally. Fourth group of animals was administered with standard drug fenofibrate 65mg/kg). All the groups of animals were measured for body weight on 5th, 10th, 15th, 20th and 25th days. After 25 days the blood was collected in heparinized tubes and to determine biochemical parameters.
Blood Sample Collection and Analysis
On the 25th day, blood was collected by retro-orbital sinus puncture, under mild ether anaesthesia, after 8 hr of fasting and allowed to clot for 30 minutes at room temperature. Blood samples were centrifuged at 3000 rpm for 20 minutes. Serum was separated and stored at -20°C until biochemical tests were estimated. A serum sample was analyzed by semi auto analyzer against the blank for cholesterol, triglyceride (TG) and high-density lipoprotein (HDL), very low-density lipoproteins (VLDL), low density lipoprotein (LDL) and calculated by using the formula [16].
Parameter Analysis
The cholesterol was estimated according to the method of [17]. Triglycerides were estimated by the method of [18]. Very low- density lipoprotein (VLDL) and low-density lipoprotein (LDL) were estimated according to the method of [19].
Calculation of LDL Cholesterol
LDL cholesterol was calculated by using the formula [20]. LDL cholesterol = Total cholesterol-(HDL cholesterol + Triglyceride/5) Plasma LDL cholesterol is expressed as mg/dl.
Histopathological Study
For histological examination, the tissues of coronary artery walls were stored in 10 % formalin. They were later sectioned using a microtome, dehydrated in graded alcohol, embedded in paraffin section and stained with haematoxylin and eosin.
Results
Body Weight Changes and Serum Parameters
There was a significant increase in body weight of high cholesterol diet rats, compared to normal rats. However, there was no change in the body weight of test group treated with Phormidium tenue (1.0 g/kg per day) (Table 1). The oral treatment also significantly reduced the levels of serum cholesterol, Triglyceride (TG), Low density lipoprotein (LDL), Very low-density lipoprotein (VLDL) and increased the level of High-density lipoprotein (HDL) in the hyperlipidemic rats nearer to standard drug (Table 2).
Table 1: Effect of ethanolic extracts of Phormidium tenue on body weight of HCD induced hyperlipidemic rats
|
Days |
Mean Body weight (gm)(% change in body weight) |
|||
|
Normal |
High Cholesterol Diet |
Ethanolic extract 100 mg/kg P. tenue |
Fenofibrate (65 mg/kg, p.o). |
|
|
0th day |
162 |
154 |
148 |
160 |
|
5th day |
174 |
162 |
161 |
188 |
|
10th day |
186 |
176 |
172 |
94 |
|
15th day |
194 |
182 |
182 |
109 |
|
20th day |
104 |
194 |
194 |
121 |
|
25th day |
112 |
211 |
208 |
136 |
Table 2: Effect of ethanolic extracts of Phormidium tenue on HCD induced hyperlipidemic rats
|
Parameter |
Normal |
High Cholesterol Diet Control |
HCD treated with Phormidium tenue (1.0 g/kg b.wt, p. o). |
HCD treated with Fenofibrate (65mg/kg, p.o). |
|
Cholesterol (mg/dl) |
68.34± 5.91a |
357.65 ±31.12b |
256.03±22.43c |
70.34±6.91a |
|
Triglyceride (mg/dl) |
71.45±6.53a |
148.92 ±12.12b |
134.36±12.33c |
84.63±7.42d |
|
HDL (mg/dl) |
29.63±2.21a |
16.81±1.12b |
26.38 ±2.31c |
25.43±2.12c |
|
LDL (mg/dl) |
22.03±1.92a |
97.95 ±8.62b |
70.00±6.71c |
27.90±2.21a |
|
VLDL (mg/dl) |
14.29±1.21a |
29.79 ±2.12b |
25.07±2.50c |
16.80±1.12d |
|
Atherogenic index |
2.30 ±0.13a |
21.27 ±1.92b |
9.70 ±0.84c |
4.55±0.31d |
Values not sharing a common superscript letter differ significantly at p < 0.05 (DMRT)
Histopathology
The coronary artery wall is divided in to several layers. The inner layer is endothelium usually smooth and unbroken. Atherosclerosis begins when the endothelium is injured. Certain white blood cells called monocytes are activated to move out of the blood stream, through endothelium of an artery in to artery’s wall. Inside endothelium, they are transferred in to foam cells, which cells collect fatty materials mainly cholesterol. The smooth muscle cells move from the middle layer in to the endothelium of the coronary artery wall and multiply there. Connective and elastic tissue materials accumulate there, as cell debris, cholesterol crystals and calcium. The accumulation of fat laden cells, smooth muscle cells and other materials forms patchy deposit called an atheroma or atherosclerotic plaque. As they grow, atheromas thicken the coronary artery’s wall and bulge in to the channel of the coronary artery. They may block a coronary artery, thereby stopping blood flow. The normal control group is shown in Figure 2 a. The coronary artery wall shows uniform size thickness with no bulging in the lumen and the endothelial cells is intact without any interruption. Also, elastic lamina and muscle fibers appear normal. All group of rats received the high fat-enriched diet for 25 days developed as atherosclerotic plaque that appeared as marked alterations in the coronary artery wall, represented by the intimal plaques as indicated by arrows in Figure 2 b.
The coronary artery wall of the group 3 rats treated with P. tenue in comparison with group 1 rats is shown in Figure 2c. Administration of fenofibrate 65 mg/kg, in addition to a hypercholesterolemia diet for 25 days completely improved the coronary artery wall which was severely disturbed by hypercholesterolemia. The coronary artery wall appears normal as in Figure 2d with no foam cells, no disturbance in the endothelial cell and no change in the muscle fibers.
Figure 1: Morphological identification Phormidium tenue
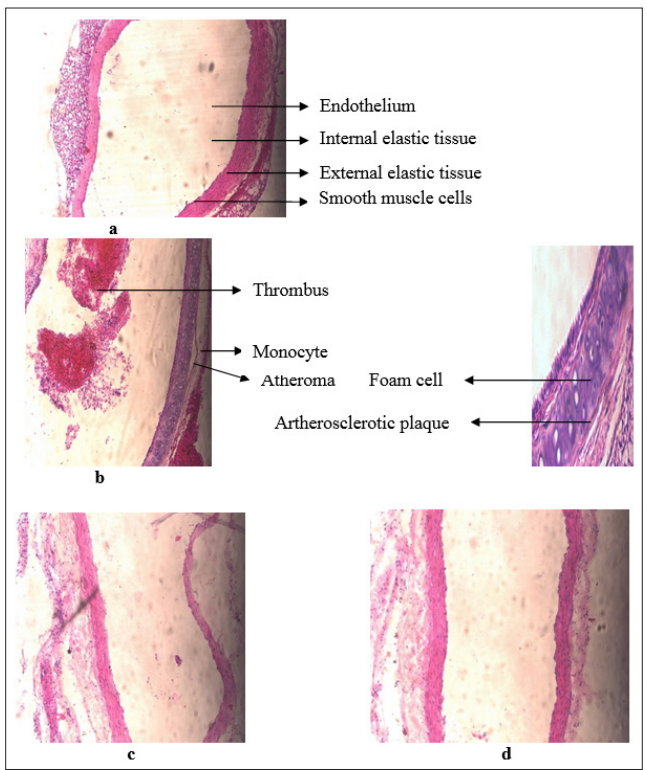
Figure 2: Histopathology of Coronary Artery wall – (a) Coronary Artery wall in Control group; (b) Coronary Artery wall in Cholesterol group; (c) Coronary Artery wall in Phormidium tenue treated; (d) Coronary Artery wall in Standard drug treated
Discussion
Hyperlipidemia, a well-known risk factor for cardiovascular disease, especially atherosclerotic coronary artery disease (CAD) is one of the major causes of premature death globally, and the most important cause of mortality in India [22]. India and World Health Organization (WHO) have been sounding an alarm on the rapidly rising burden of cardiovascular disease (CVD) for the past 15 years [23]. The reported prevalence of coronary heart disease (CHD) in adult surveys has risen four-fold in 40 years and even in rural areas the prevalence has doubled over the past 30 years. In 2005, 53% of the deaths were on account of chronic diseases and 29% were due to cardiovascular diseases alone [24]. It is estimated that by 2020, CVD will be the largest cause of disability and death in India. The country already has more than 40.9 million people with diabetes and more than 118 million people with hypertension, which is expected to increase to 69.9 and 213 million respectively, by 2025 unless urgent preventive steps are taken. WHO estimates that India lost 9 billion dollars in national income from premature deaths due to heart disease, stroke and diabetes in 2005 [25].
Indians are known to succumb to high blood pressure and heart attacks 5-10 years earlier than their Western counterparts [26]. Unfortunately, scientific data also show that socio-economically disadvantaged sections of the populations are now the dominant victims of CVD [27]. There is also preliminary evidence that the burden of CVD in rural areas is increasing [28]. Cholesterol is the building block for cell membrane and a precursor of steroid hormones. It forms several distinct particles with lipoproteins, mainly high-density lipoproteins (HDL), low density lipoproteins (LDL), and very low-density lipoproteins (VLDL). It is well established that levels of LDL and VLDL cholesterol are atherogenic whereas HDL cholesterol has protective effects on the development of atherosclerosis [29]. Increased levels of LDL and VLDL are the major independent risk factors for cardiovascular events, whereas low level of HDL and elevated triglycerides (TG) are also recognized as residual risks for cardiovascular diseases [30].
The hypolipidemic effect of marine cyanobacteria, P. tenue has not been previously reported. However, several studies have reported on cholesterol-regulatory properties of microalgae (Spirulina and Chlorella) [31]. In rat, mice and human studies, Spirulina showed positive effect with respect to serum cholesterol reduction and the elevation of HDL-cholesterol level and HDL to LDL ratio [32- 33]. Hence the present study was undertaken to evaluate the anti- hyperlipidemic effect of a marine cyanobacterium, Phormidium tenue. In this study, oral treatment of P. tenue at 1.0 g/kg per day, significantly reduced the level of serum cholesterol, TG, LDL & VLDL and increased the HDL level in hyperlipidemic rats nearer to standard drug (Table 2). This finding is in support of earlier studies, that Spirulina supplemented at 5, 10 and 15% of the diets have caused a significant inhibition of total HDL-cholesterol and TG hypolipidemic rats [33-34] and the supplementation of 0.5 g/day of S. platensis has caused a decrease in the induced hypercholestearolemia in rabbits. The serum levels of cholesterol has decreased in the rabbits fed a hypercholesterolemic diet without S platensis in comparison to those fed a hypercholesterolemic diet supplemented with S. platensis. The serum levels of HDL are higher in the groups fed with Spirulina [35]. The hypolipidemic effect of Spirulina or its extracts has been demonstrated in various animal models including mouse, rat, hamster, and rabbit. The cholesterol lowering activity of Spirulina is reported for the first time in albino rats [36], followed by that in mice [36,28]. In the mouse study, supplementation of 16% Spirulina in a high fat and cholesterol diet has resulted in a significant reduction in total serum cholesterol, LDL, VLDL cholesterol, and phospholipids whereas serum HDL cholesterol is concurrently increased. In addition, high hepatic lipids induced by the high fat and cholesterol diet are markedly reduced by Spirulina consumption [28].
A possible mechanism for lowering plasma cholesterol in animals fed on diet containing cyanobacteria is that caused a reduction of lipid peroxidation of endoplasmic reticulum which is the site of lipoprotein synthesis. A second possible mechanism for lowering plasma cholesterol concentration in animals fed on diet containing p tenue is as follows: carotenoids of the cyanobacteria inhibit the cholesterol synthesis through the inhibition of β-hydroxy- β-methylglutaryl CoA (HMG-CoA) synthesis. This enzyme involves in cholesterol biosynthesis, which is expected to parallel to the activity of HMG-CoA reductase the rate limiting enzyme in cholesterol biosynthesis. However, the work of [37] have showed that hypocholesterolemic effect of lycopene and β- carotene is related to suppression of cholesterol synthesis by inhibiting of β-hydroxy-β-methylglutaryl CoA (HMG-CoA) reductase and to augmentation of low-density lipoprotein (LDL) receptor activity in macrophage. Also, [38] has found that rats fed 1 % dietary astaxanthin for 30 days increase HDL cholesterol (good cholesterol) of 57 mg /dl compared to the control diet with 42.4 mg/dL. Conversely, the low-density lipoprotein (LDL) decreases from control diet of 12.5 mg/dl to 9.6 mg/dl when supplemented with astaxanthin. Hence, P. tenue carotenoids containing β-carotene, lycopen, astaxanthin and cryptoxanthin have significant hypocholesterolemic effect.
Conclusion
Major limitations in the effective pharmacological treatment of hyperlipidemia are the constraints imposed on health care resources. In this scenario, the problem tackled by use of natural agents of marine cyanobacterium Phormidium tenue due to its cost effectiveness and minimal side-effects. On the basis, the study investigated the anti-hyperlipidemic activity of formulated high cholesterol diet (Cholesterol 2%, Sodium cholate 1% and coconut oil 2%, with standard powdered animal food) induced hyperlipidemic in Wistar rats. The experimental hyperlipidemic rats were treated with Phormidium tenue at a dose 1.0 g/kg per day and Fenofibrate was used as standard drug. Phormidium tenue indicated significant reduction in the levels of serum cholesterol, triglycerides (TG), low density lipoprotein (LDL) and very low-density lipoprotein (VLDL) and increased the high-density lipoprotein (HDL) level 1.0 g/day against hyperlipidemic rats. The histopathological studies revealed that the coronary artery wall appeared normal, with no foam cells, no disturbance in the endothelial cell and no change in the muscle fibers in normal and Fenofibrate treated rats. The coronary artery wall of the group 3 rats treated with P. tenue was related to group 1 rats. This study suggests that P. tenue has the potential protective role for coronary heart disease.
Acknowledgments
The authors are thankful to Annamalai University and Thanthai Hans Roever College authority for providing infrastructure facilities.
References
- Jain KS, Kathiravan MK, Somani RS, Shishoo CJ (2007) The biology and chemistry of hyperlipidemia. Bioorganic and Medicinal Chemistry 15: 4674-4699.
- Goodman, Gilman Ed (2001) The pharmacological basis of therapeutics:10th Edn, McGraw hill medical publishing division, New York 242-265.
- Saravana Kumar A, Vijit Mazumder A, Saravanan VS (2008) Antihyperlipidemic activity of Camellia sinensis leaves in Triton WR-1339 induced albino rats. Pharmacognosy Magazine 4: 60- 64.
- Pratico D (2005) Antioxidants and endothelium Atherosclerosis 181: 215-224.
- Joshi P, Islam S, Pais P (2007) Risk factors for early myocardial infarction in South Asians compared with individuals in other Journal of the American Medical Association 297: 286-294.
- Nakaya N, Homa Y, Goto Y (1988) Cholesterol lowering effect of Nutrition Reports International 37: 1329- 1337.
- Becker EW, Jakober B, Luft D (1986) Schmuling Clinical and biochemical evaluations of the algae Spirulina with regards to its application in treatment of obesity: A double blind cross over Nutrition Reports International 4: 565-574.
- Devi M A, Venkataraman LV (1983) Hypocholesteromic effect of blue green algae Spirulina platensis in albino rats. Nutrition Reports International 28: 519- 530.
- Mani UV, Desai S, Iyer U (2000) Studies on the long- term effect of Spirulina supplementation on serum Lipid profile and glycated proteins in NIDDM patients. Journal of Nutraceuticals, Functional and Medical Foods 2: 25-32.
- Bergman MB, Kranjcevic K, Reiner Z, Milakovic BS, Stojanovic S, et al. (2005) Drug therapy of cardiovascular risk factors: guidelines versus reality in primary health care service. Croatian Medical Journal 46: 984-989.
- Oluwatosin A, Olajumoke A, Jonah A, Michael AF (2008) Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vascular Health and Risk Management 4: 235-241.
- Rippka R, Deruelles J, Waterbury JB, Herdman H, Stainer SY (1979) Generic assignments, strain histories and properties of pure culture of cyanobacteria. Journal of General Microbiology 111: 1-61.
- Palaniselvam V (1998) Epiphytic cyanobacterial of mangroves: ecological, physiological and biochemical studies and their utility as biofertilizer and shrimp - Ph.D.Thesis, Annamalai University, India. pp 141.
- Desikachary TV (1959) Cyanophyta, Indian Council of Agricultural Research, New Delhi pp 686.
- Humm J, Wicks R (1980) Introduction and guide to the marine blue - green algae. A a wiley-interscience publication,New York pp.1-273.
- Dikshit M, Rachh PR, Nayak BS, Shah BN, Modi KP, et al (2009) Antihyperlipidemic acitivity of Syzygium cumini seed extract on high cholesterol fed diet International Journal of Pharmaceutical Science 1:330-332.
- Zlatkis A, Zak B, Boyle AJ (1953) Journal of Laboratory and Clinical Medicine 41: 486.
- Foster JB, Dunn RT (1973) Stable reagents for determination of serum triglycerides by colourimetric Hantzsch condensation Clinical Chemistry 19: 338-340.
- Aaamann G, Schriewer H, Schmitz G, Hagele EO (1983) Quantification of high-density lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2.. Clinical Chemistry 29: 2026-2030.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of LDL cholesterol in plasma, without use of the preparative ultra- centrifuge. Clinical Chemistry 18:499-502.
- Prasanna M (2000) Hypolipidemic effect of Funugreek: a clinical study. Indian Journal of Pharmacy and Pharmacology 3234-3236.
- Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and Chemico-Biological Interactions 167: 219-226.
- Reddy KS (1993) cardiovascular disease in World Health Stat Q 46:101-107.
- Reddy KS, Shah B, Varghese C, Ramadoss A. (2005) Responding to the challenge of chronic diseases in India. Lancet Journal 366:1744 -1749.
- Arvind Malik (2012) Reducing risk of cardiovascular disease through physical activities. International Journal of Multidisciplinary Management Studies 2: 65-69.
- Mohan V, Sandeep S, Deepa R, Shah B, Varghese C (2007) Epidemiology of type 2 diabetes: Indian scenario. Indian Journal of Medical Research 125:217- 230.
- Gupta DK, Verma LK, Khosla PK, Dash SC (1991) The prevalence of micro albuminuria in diabetes:a study from North India. Diabetes Research and Clinical Practice 12: 125-128.
- Joshi R et al (2006) Chronic diseases now a leading cause of death in rural India - mortality data from the Andhra Pradesh Rural Health Initiative. International Journal of Epidemiology 35: 1522-1529.
- Reddy KS (1998) Rising burden of cardiovascular disease in India. In: Sethi KK, editor. Coronary artery disease in Indians: a global Mumbai: Cardiological Society of India, pp. 63-72.
- Belay A (2002) The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. The Journal of the American Nutraceutical Association 5: 27- 48.
- Fong B, Cheung M, Lee M (2000) Effect of dietary Spirulina on plasma cholesterol and triglyceride levels in In: 4th Asia Pacific Conference on Algae Biotech pp 150.
- Ramamoorthy A, Premakumari S (1996) Effect of supplementation of Spirulina on hypercholesterolemic Journal of Food Science and Technology 33:124-128.
- Iwata K, Inayana T, Kato T(1991) Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose- induced hyperlipidemia in rats. Journal of Japan Society of Nutririon and Food Sciences 40:463-467.
- Jewell C, O’Brien NM (1999) Effect of dietary supplementation with carotenoids with on xenobiotic metabolizing enzymes in the liver, lung, kidney and small intestine of the British Journal of Nutrition 81: 235-342.
- Luciane MC, Ana Luiza Muccillo-Baisch, Jorge Alberto Vieira Costa (2008) Spirulina platensis Effects on the Levels of Total Cholesterol, HDL and Triacylglycerols in Rabbits Fed with a Hypercholesterolemic Diet, Brazilian Archives of Biology and Technology 51:405-411.
- Rastogi T et al (2004) Diet and risk of ischemic heart disease in India. American Journal of Clinical Nutrition 79: 582-592.
- Fuhrman B, Elis A, Aviram M (1997) Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochemical and Biophysical Research Communications 233(3):658-662.
- Murillo E (1992). Cholesterolemic effects of canthaxanthin & astaxanthin in rats. Archivos Latinoamericanos de Nutricion 42: 409-13.

