An Overview of an Invasive Fungal Infection In Covid-19: Mucormycosis
© 2021 Himanshu, Mukesh kumar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Mucormycosis is an invasive fungal infection of zygomycetes class, comprised the orders of entomophtherales and mucorales which is common infection in immuno compromised patients, especially in stem cell transplantation, hematological malignancy and diabetes mellitus. It is the third invasive fungal infection after aspergillosis and candidiasis both strain belong the class of zygomycetes. Mucormycosis generally transmitted in humans by the inoculation and inhalation of spores in mucous membrane as well as skin. After the deadly outbreak of corona virus, this infection has taken a different form in those patients who suffer from the covid-19 which is categorized as a post covid-19 disease. Aim of this review to highlight the recent awareness about invasive fungal treatment.
Introduction
At the end of December 2019, a deadly outbreak of corona virus occurred in Wuhan, China which spread rapidly in all over the world. With the help of exploitation of sequence technology corona virus disease gets a discontiguous identification commonly known as corona virus disease 2019 and the etiological agent was elucidated as Severe acute respiratory syndrome. COVID-19 or SARS-CoV-2 generally spread or transmit via respiratory droplets between human-to-human and it also consisting different types of symptoms such as pharyngitis, sternutation, cold, headache, tightness etc [1]. If we concern about the current status of weekly epidemiological update of covid-19 given by world health organization till end of June 3rd 3 million cases were reported in a week with 60 thousand mortality rate which is shown in (Table 1).
Table 1: Reported cumulative COVID-19 confirmed cases and mortality rate as per world health organization
|
WHO Region |
New cases in last 7 days (%) |
Changes in new cases in last 7 days |
Cumulative cases (%) |
New deaths in last 7 days (%) |
Change in new deaths in last 7 days |
Cumulative deaths (%) |
|
America |
1173561 (36%) |
-5% |
53937714 (44%) |
31040 (51%) |
2% |
1299243 (48%) |
|
Europe |
1441065 (44%) |
13% |
4251672 (35%) |
21772 (36%) |
1% |
929332 (34%) |
|
South-East Asia |
298438 (9%) |
49% |
14182826 (12%) |
2435 (4%) |
14% |
214790 (8%) |
|
Eastern Mediterranean |
263650 (8%) |
8% |
7124121 (6%) |
3253 (5%) |
12% |
153446 (6%) |
|
Africa |
50916 (2%) |
-3% |
2999152 (2%) |
1428 (2%) |
10% |
76113 (3%) |
|
Western Pacific |
63730 (2%) |
29% |
1775560 (1%) |
486 (1%) |
-33% |
30843 (1%) |
|
Global |
3291360 (100%) |
8% |
122536880 (100%) |
60414 (100%) |
3% |
2703780 (48%) |
Mocormycosis and zygomycosis is an invasive fungal infection belonging to the order mucorales generally caused by a group of molds called mucormycetes [2]. Mocormycosis is the second frequent invasive mold disease after aspergillosis. Patients have higher risk who suffers from poorly controlled diabetes mellitus, those who undergoing the treatment of hematopoietic stem cell transplantation, treatment of hematological cancer and who have sustained severe trauma to soft tissues [3]. These types of conditions referred as black fungus. As per the survey that mocormycosis is rising in post covid-19 patients and it comes when the patients having low immunity power and have been wearing a mask which is used in multiple times, it will absorbed humidity by the process of inhalation and exhalation and will get a proper atmosphere to grow fungus [4].
History
An American pathologist R.D Baker coined the term mocormycosis for the first time which is also known as zygomycosis. It is defined as an invasive fungal infection caused by mucorales and the number of zygomycotic species [5]. Mucormycotina are the common example of saprobes which originates from soil or decaying matter. In 1885, the German pathologist Paltauf reported the first case of mocormycosis and it was accrescent seen between the immuno compromised individuals during 1980s and 1990s [6]. According to the proliferation rate, a case study carried out in France which reported 7.4% amplification per year and worldwide occurrence has been reported along with mucorales infection with the possibilities of their seasonal variation [7, 8].
Microbiology
Fungi belong to order mucorales which differentiate into six categories i.e. Mucoraceae, Cunninghamellaceae, Syncephalastraceae, Saksenaceae, Mortierellaceae and Thamnidaceae shown in (Table 2).
Table 2: Classification of the Etiological agents responsible for mocormycosis
|
Sr. No. |
Family |
Genus |
Species |
|
1 |
Mucoraceae |
Absidia |
A. Corymbifera |
|
Apophysomyces |
A. elegans |
||
|
Mucor |
M. circinelloides |
||
|
M. hiemalis |
|||
|
M. racemosus |
|||
|
M. ramosissimus |
|||
|
M. rouxianus |
|||
|
Rhizopus |
R. pusillus |
||
|
R. arrhizus |
|||
|
R. azygosporus |
|||
|
2 |
Cunninghamellaceae |
Cunninghamella |
C. bertholletiae |
|
3 |
Mortierellaceae |
Mortierella |
|
|
4 |
Saksenaceae |
Saksenaea |
S. vasiformis |
|
5 |
Syncephalastraceae |
Syncephalastrum |
S. racemosum |
|
6 |
Thamnidaceae |
Cokeromyces |
C. recurvatus |
Mucorales can be grows in both selective and non-selective media. It grows rapidly and just takes few takes with mycelia elements expanding to cover the entire plate. The mycelium is fibrous and ‘cotton candy like’ in appearance and its growth is so vigorous that the group demonstrated as lid filter. Recognition of the agents is amenable for mocormycosis is based on their microscopic and macroscopic morphological criteria, carbohydrate assimilation and optimum temperature which are favorable for its growth [9]. Macroscopic criteria are accessory in establishing a presumptive identification which is confirmed by microscopic dissection after staining[10].
Crucial macroscopic characteristics are vigorous growth, hyaline appearance, light coloration on the other side of the plate (tan to yellow for more species) and variable degree of coloration on the sporulating surface of the colonies (from pure white to tan, gray, brown or even black) [11]. The family Mucoraceae can be categorized on the basis of their morphology of the predominant asexual spore producing structures (sporangiola producers, sporangium producers and merosporangium producers) [12]. Species can be disassembled by elements such as rhizoids, columella and stolons which are ordinarily visualized in the microbiology laboratory on lactophenol cotton blue slides [13]. Hypae are wide, non-septate and measures 10-20 μm in diameter, with branches which is separated from the main body nearly at 90° angles shown in (Figure 1).
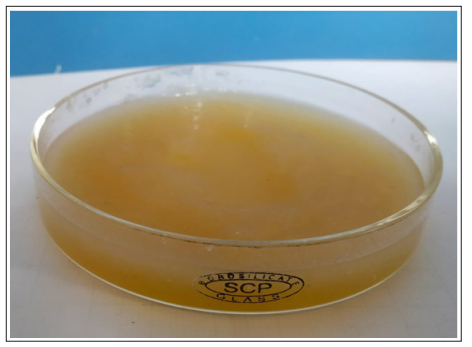
Figure 1: Macroscopic appearance of a positive culture for mucorales
Risk Factors
Most of the patients with invasive mocormycosis have several underlying diseases that are both predispose and influence the clinical presentation. The most common underlying diseases are [14].
- Diabetes mellitus
- Hematopoietic cell transplantation
- Hematologic malignancies
- AIDS
- Solid organ transplantation
- Iron overload
- Malnutrition
- Trauma and burns
Pathogensis
Mucorales strikes on the deep tissues through the inhalation of airborne spores, percutaneous inoculation or ingestion. They settle a high number of patients but don’t inevitably cause invasion [15]. Once the spores have perforated to subcutaneous tissues or lungs, they are converging by the first line of safeguard, mononuclear and polynuclear pahgocytes. The pahgocytes are able to kill the spores of mucorales in the host by generating oxidative metabolites [16]. There is a high risk of mocormycosis for those who are severely immune compromised neutropenic patients and those with phagocyte dysfunction e.g. hyperglycemia. Ketoacidosis decreases the agitation of these pahgocytes as regards the source of infection and their competence for lysis by oxidative and non- oxidative mechanism [17]. High concentration of iron in the serum is another risk-factor for mocormycosis. Patients treated with deferoxamine have a high instance of mocormycosis. Perhaps, because mucorales use this chelant as a siderophere to get more iron [18]. It is well-known that delivering of deferoxamine or iron to the animals infected with mucorales reduces the survival rate and the higher risk of mocormycosis in patients with ketoacidosis may also be due to the release of iron bound to proteins [19] If the phagocytes are escaped by the spores, they can invade vessels, in part by effective adherence to the endothelial cells ability that Rhizopus oryzae preserves even when the fungus is not viable [20].
Symptoms
These are the various sing and symptoms in mocormycosis such as fever, swelling over the nose and eye, headache, shortness of breath, vomiting, cough, abdominal pain, skin swelling, skin redness, bloody vomit, skin redness, dark scabbing in the nose etc [21]. Graphical representations of mocormycosis symptoms was shown in (Figure 2).
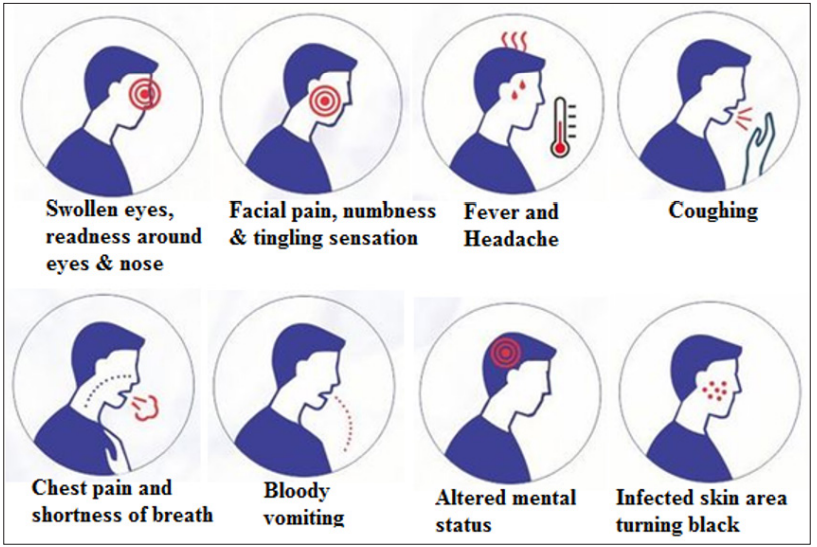
Figure 2: Graphical representations of mocormycosis symptoms
Diagnosis
Diagnosis of mocormycosis comprises cautious evaluation of clinical manifestations utilization of computed tomography, magnetic resonance imaging modalities, finest application of clinical microbiological technique, and specialist assessment of cytological and histological provision and execution of molecular detection. Investigation of host factors allow considerably to the estimation of a patients possibility for an invasive fungal infection known as mocormycosis. PAS stains, histopathological examination, direct culture examinations are the various types of laboratory techniques for detecting mocormycosis [22].
Treatment
Mocormycosis treated by using antifungal drugs usually isavuconazole, posaconazole and Amphoteriocin-B-B and some other drugs are not effective against the fungi which cause mocormycosis such as voriconazole, fluconazole and echinocandins [23-27] Antifungal strategic for mocormycosis shown in (Table 3).
Table 3: Antifungal strategic for mocormycosis treatment
|
Therapy |
Antifungal |
Pors |
Cons |
|
Established therapies |
Amphoteriocin-B-B deoxycholate (AmB) |
50 years experience Toxicity cidal |
Toxicity |
|
Liposomal Amphoteriocin-B-B (LAmB) |
Less toxic than AmB Improved CNS penetration High-dose LAmB (15 mg/kg/day) superior to AmB (1 mg/kg/day) in murine model Superior to AmB in retrospective clinical study |
Resistance seen in individual isolates Most expensive polyene |
|
|
Amphoteriocin-B B lipid complex (ABLC) |
Less toxic than AmB |
Inferior CNS penetration vs. LAmB in one rabbit study Not superior to placebo or AmB in murine model even at high doses (up to 30 mg/kg/day) No comparative clinical data published |
|
|
Investigational/adjunctive therapies |
Itraconazole |
Superior toxicity profile Successful case reports |
Poor activity in animal models despite in vitro susceptibility Breakthrough mocormycosis described during prophylactic itraconazole |
|
Posaconazole |
More effective than itraconazole in animal models Successful case reports Possible combination with polyene therapy, but no data available |
Not yet FDA approved Static in vitro Activity inferior to AmB in murine Models |
|
|
Caspofungin |
Very low toxicity Synergistic with ABLC in murine model FDA approved (not for mocormycosis) |
Virtually no clinical data for mocormycosis Minimal activity as mono therapy in murine model |
|
|
|
Iron chelation |
Theoretical benefit in combina- tion with antifungal |
No data available No effective agents are FDA approved |
|
|
Hyperbaric oxygen |
Nontoxic Successful case reports |
Not widely available No controlled studies |
|
Cytokine therapy |
In vitro activity Successful case reports |
Limited data Expensive Toxicity profile unclear |
Preventions
There are some necessary precaution against the mocormycosis such as [28-29].
- Use face mask if you are visiting any construction sites
- Maintain personal hygiene.
- Wear shoes, long sleeve shirts, long trousers and gloves during gardening
- Mocormycosis can be managed by controlling diabetes, reducing the quantity of steroids and discontinuing immunomodulating drugs.
- Control hyperglycemia.
- Monitoring the blood glucose level at particular time interval.
- Take antifungal and antibiotics judiciously
Prognosis
Prognosis generally depends on the limitation of demonstration of the disease and emphatic treatment begins in the response to the diseases. The survival rate of rhino-cerebral disease in patients without systemic disease is near about 75% with other disease is near about 20% and in pulmonary disease it is contemplated to be fatal [30]. Survival rate varies with foci of the infection: cerebral mocormycosis-45%, pulmonary forms-36%, focal cerebral mocormycosis-33%, cutaneous isolated-90%, sinusitis without cerebral involvement-87%, disseminated disease-16% and involvement of gastro intestinal form-10%. Preferable survival rate can be instated in patients with low baseline serum concentration of iron/ferritin, malignant cases and neutropenia which is not associated with infection [31].
Conclusion
In today’s era mocormycosis or black fungus is a life threatening invasive fungal infection which becomes a very serious problem in all over the world after the deadly outbreak of corona virus. According to the prospective surveillance among 16,808 transplant remittees featured in 23 institutions during 2001-2006 found that mocormycosis was the extreme usual type of invasive fungal infection in stem cell remittees and accounted for 8% of all invasive fungal infection and a review of published it found an overall cause mortality rate of 54%. The mortality rate varied on the basis on the underlying patient condition which affected the body sites by the various types of fungus. The way out of this disease is, if someone found the symptoms which show a disease like mocormycosis then contact to the physicians and health care providers to tackle this terrible disease and take those precautions which are helpful for us to fight against this disease.
References
- Van Der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, et al. (2004) Identification of a new human coronavirus. Nature medicine. 10: 368-373.
- Kerbaul F , Guidon C, Collart F , Hubert Lépidi, Bruno Cayatte et al. (2004) Abdominal wall mocormycosis after heart J Cardiothorac Vasc Anesth. 18: 822- 823.
- Becker BC, Schuster FR, Ganster B, Seidl HP , Schmid I, et (2006) Cutaneous mocormycosis in an immunocompromised patient. Lancet Infect Dis 6: 536.
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT , Kontoyiannis DP et (2012) Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary and disseminated mocormycosis (zygomycosis). Clin Infect Dis. 54: S55-S60.
- Kwon-Chung KJ (2012) Taxonomy of fungi causing mocormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic Clinical Infectious Diseases54(suppl_1): S8-15.
- Mohammadi R, Nazeri M, Sayedayn SM, Ehteram H. A successful treatment of rhinocerebral mocormycosis due to Rhizopus Journal of research in medical sciences: The Official Journal of Isfahan University of Medical Sciences, 2014; 19(1): 72.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, et al. (2012) Epidemiology and clinical manifestations of mocormycosis. Clinical Infectious Diseases. 54: S23-34.
- McNulty JS (1982) Rhinocerebral mocormycosis: predisposing factors. Laryngoscope 92: 1140.
- Lee FY, Mossad SB, Adal KA (1999) Pulmonary mocormycosis: the last 30 Arch Intern Med . 159: 1301.
- Ismail MH, Hodkinson HJ, Setzen G, C Sofianos, M J Hale, et
- (1990) Gastric mocormycosis. Trop Gastroenterol. 11: 103.
- Adam RD, Hunter G, DiTomasso J, Comerci G Jr (1994) Mocormycosis: emerging prominence of cutaneous Clin Infect Dis. 19: 67.
- Cocanour CS, Miller-Crotchett P, Reed RL 2nd, P C Johnson, R P Fischer, et (1992) Mocormycosis in trauma patients. J Trauma. 32: 12.
- Levy E, Bia MJ (1995) Isolated renal mocormycosis: case report and review. J Am Soc Nephrol. 5: 2014.
- Prabhu RM, Patel R (2004) Mocormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 10: 31-47.
- Waldorf AR (1989) Pulmonary defense mechanisms against opportunistic fungal Immunol Ser. 47: 243-271.
- Chinn RY, Diamond RD (1982) Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 38: 1123-1129.
- Boelaert JR, de Locht M, Van Cutsem J, V Kerrels, B Cantinieaux, et (1993) Mocormycosis during deferoxamine therapy is a siderophoremediated infection. In vitro and in vivo animal studies. J Clin Invest. 91: 1979-1986.
- Abe F, Inaba H, Katoh T, Hotchi M (1990) Effects of iron and desferrioxamine on Rhizopus infection. Mycopathologia. 110: 87-91.
- Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev. 13: 236-301.
- Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE Jr et (2005) Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 73: 778-783.
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP, et (2012) Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mocormycosis (zygomycosis). Clinical Infectious Diseases. 54: S55-60.
- Groll A H, N Giri, V Petraitis, R Petraitiene, M Candelario, et al. (2000) Comparative efficacy and distribution of lipid formulations of amphoteriocin-B B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182: 274-282.
- Ibrahim A S, V Avanessian, B Spellberg, J E Edwards (2003) Liposomalamphoteriocin-BB,andnotamphoteriocin- BBdeoxycholate,improves survival of diabetic mice infected with Rhizopus oryzae. Agents Chemother. 47: 3343-3344.
- Gleissner B, A Schilling, I Anagnostopolous, I Siehl, E Thiel et (2004) Improved outcome of zygomycosis in patients with hematological diseases? Leukemia Lymphoma 45: 1351–1360.
- Ibrahim A S, S Klein H Lee, Y Fu, H Waskin, J Edwards, et al. (2000) Presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto,
- Spellberg B, Y Fu, J Edwards Jr, A S Ibrahim. (2005) Combination therapy with amphoteriocin-B B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic Antimicrob. Agents Chemother. 49: 830-832.
- Eisen D P, J Robson (2004) Complete resolution of pulmonary Rhizopus oryzae infection with itraconazole treatment: more evidence of the utility of azoles for zygomycosis. Mycoses 47: 159-162.
- Pellberg B, Edwards J, Ibrahim A (2005) “Novel perspectives on mocormycosis: pathophysiology, presentation, and management”. Clin. Microbiol. Rev. 18 : 556-569.
- Brizendine KD, Vishin S, Baddley JW (2011) Antifungal prophylaxis in solid organ transplant recipientsexternal Expert Rev Anti Infect Ther. 9: 571-581.
- Rogers TR, Slavin MA, Donnelly JP (2011) Antifungal prophylaxis during treatment for haematological malignancies: are we there yet? external iconBr J 153: 681-697. Spellberg B, Ibrahim A, Rolides E, Lewis RE, Lortholary O, et al. (2012) Combination therapy for mocormycosis: why, what, and how?. Clinical infectious diseases. 54: S73-8.

