Effects of Water Hyacinth (Eichhnornia Crassipe) on Carbon and Phosphorus Mineralization in Wetland Soils
© 2024 Grace Otobo, Aduaka Cecilia Nkemjika, Endurance Fegor Isoje, Vinna Daniel Chukwuna, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This work investigated the kinetics of Carbon and Phosphorus Mineralization in Wetland Soils amended with water Hyacinth (Eichhnornia Crassipe). The work studied the effects of water hyacinth also known as Eichhornia Crassipe on Carbon and Phosphorus Mineralization in Delta Wetland soil. The kinetics of Carbon and Phosphorus Mineralization was studied using Pseudo Second Order Equation. The Respiration Assay of the organic matter (Water Hyacinth) incorporated leads to emission of carbon in the form of carbon (iv) oxide and greater degree of microbial activities than those occurring in the control Soils after initial stage of incubation. Reaction Rates decreases with time in the amended Soil. In all the Soil samples, initial rapid phase of mineralization was clearly observed. Phosphorus Mineralization increased gradually with time in the amended soil as a result of high degree of organic matter and the pH of the soils.
Introduction
Phosphorus is one of the major plant nutrients in the soil. It is a constituent of plant cells essential for cell division and development of the growing tips of plant. Low availability of phosphorus is often limiting plant growth in most soils [1,2].
The mineralization of organic matter in soil called decomposition which is characterized by decrease in organic matter contents and increase of available mineral previously immobilized in organic matter in the soil depends on different factors which include Soil types, climate condition and the quality of the organic matter incorporated [3,4].
All heterotrophic microorganisms are capable of decomposing organic matter and this decomposition process has been used to indicate biological state of soil [5].
Organic carbon amendment through provision of available carbon to organic matters triggers the growth of microorganism, the growth of bacteria and fungi, stimulating Phosphorus immobilization, initially followed by Phosphorus release from necrotic microbial biomass [6,7].
Water hyacinth (Eichhornia Crassipe) a problematic invasive plant that clog water ways in warm temperate region, an alien to Nigeria Ecosystem is a native of south America and was first introduced in 1884 as an ornamental plant in the US [8].
Water hyacinth has been used in various places in the world, in making furniture, handbags, basket, ropes, household goods and interior products such as lamp shield, picture frame by Non Governmental organization and Entrepreneurs Ware hyacinth is a good source of organic matter for composting organic farming [9-11]. It is used internationally for organic fertilizer, animal feeds and silage for Cattles, Sheep, Geese, Pigs and other lives stocks feeds the high moisture content of water hyacinth adding so much to handling cost tends to limit commercial ventures [10,12,13].
In Nigeria, water hyacinth has been seen by the Fishermen and Sailors as problematic plants due to fast invasion of this plant on the Ecosystem, thereby reducing other population bio diversity. As a result many fishes die due to lack of oxygen and sunlight. As a way of management of the water body and improvement of the wetland soils also regarded as non agricultural land, it is important to recycle organic material such as water hyacinth into the Soil. Recycling of organic material will improve soil conditions such as soil development, structural maintenance, improvement of physical properties, decrease susceptibility to erosion, encouragement of microbial activities, and provision of potentially available plant nutrient. Removal of this water hyacinth will also improve the water body condition such as increase in oxygen, sun light etc.
The effect of water hyacinth when incorporated into wetland soil in relation to Phosphorus and Carbon mineralization and microbial activities were described in this work. This work is meant to investigate the usefulness of water hyacinth as organic manure in wetland soil of Niger Delta.
Sampling Area
Three different soils samples were collected from three different Wetland areas in Delta State. Soil samples for the study were made up of Fadama soil collected from latitude 5 045 1N (Uwherun) and longitude 6 071 E, Enitsol soil collected from latitude 50 15 1N and longitude 60 111E (Patani) and acid Sulphate collected from latitude 50 36 1N and longitude 50 46 1E. (Warri)
Physical Properties of Soil Samples
The Fadama Soil which was collected from Uwherun is grey colour, Sandy about 85% Sand, 5% Silt and 10% Clay, feels gritty when rubbed between fingers, not plastic or sticky when moist. The Acid Sulphate Soil is black Sandy loam Soil with 65% Sand 20% Silt and 15% Clay. It feels a bit smooth when rubbed between hand, powdery and not sticky when moist.
Enitsol Soil is yellow Clay Soil with 55% clay, 32% Silt and 13% sand. It feels smooth, sticky and plastic, when dry.
Materials and Methods
The three soil samples [Fadama (FA), Enitsol (ES) and acid Sulphate (AS)] collected from Uwherun, Patani and Warri respectively were selected to represent the major soil series in the Niger Delta and include a wide range of chemical properties. Samples which are of field moist soil were brought to the laboratory, passed through a 2mm sieve and was placed in a polythene bag and stored in a refrigerator at 40 C until incubation time. A sub- sample of this was used in the incubation experiment to study the mineralization of Phosphorus and Carbon. 1000grams of each soil samples were weighed into the incubating container. To these containers were added the organic waste fractions (Eichhornia Crassipe) 40grams for 56 days. De-ionized water was added to each soil mixture to bring it to 60% of its water holding capacity for each soil sample. Throughout the incubation period water losses exceeding 10% of the initial value were compensated by adding fresh distilled water. After every 14 days of incubation 10grams of the mixture we removed from each soil mixture and air dried before they were kept in a clean container which were well labeled awaiting analysis [14].
The second portion of the soil sample was air dried at room temperature for 48 hours and stored in a tightly sealed bottle. A sub sample of air dried portion was ground to pass 80 mesh sieve (180mm) for determination of chemical properties [14]. The organic matters (Eichhornia Crassipe) was oven dried for 3 days at 650C and ground to pas 20 mesh sieve (850mm) and was shared in a glass bottle at 40oC.
The pH of the samples was determined as described by Ademoroti.10gramsof soil samples mixtures were weighed into 200ml beaker. 20 ml of distilled water was added and allowed to stand for 30 minutes. The pH meter which was standardized with buffer pH4 and pH9 was used. The Total organic carbon (TOC) of the soil samples were determined using Walkley and Black method. 10 ml of 0.5 K2Cr2O7 was added to one gram of each soil mixture and swirled gently. To each, 20ml of conc. H SO was added immediately and allowed to stand for 20minutes. Each was diluted to 250ml mark. 10 ml Conc. H3PO5 and 0.5% of diphenylamine were added and blue colour appears. The chromic acid not used up in oxidation was titrated with Iron (II) ammonium tetraoxosulphate (vi) hexahydrate from burette. The solution changed from blue to green at the end point. Blank titration was carried out [15-16].
The emitted CO2 in the incubated mixtures which was captured in 0.1M NaOH was measured volumetrically with 0.1M HCl in the presence of Barium Chloride solution and phenolphthalein as an indicator [17]. All reagents used were of analytical grade obtained from Sigma BDH and Bulk Scientific.
Determination of Total Phosphorus and Available Phosphorous The total and available Phosphorus in the soil were determined by using phospho-vanado-molybdate method. 2grams of air dried Soils mixtures were shaken with 400ml of buffer in a stopped bottle for 30 minutes. The filtrates were taken and diluted. Paranitrophenol and ammonia solution were added drop wise until yellow colour appeared. The colour was discharged by adding a drop of HCl. 4ml of ammonium molybdate sulphate and mixed properly.0.5M of stannous chloride solution was added and diluted to 100ml. the readings were taken within 10-15 minutes. The same way a blank process was carried out [18].
Results and Discussion
The three-soil sample (Fadama, Enitsol and acid sulphate soil) pH values before amendment are moderately acidic with pH values of 6.29, 5.75 and 5.5 respectively which correspond to the pH of the soil in Niger Delta area [19,20]. Addition of organic wastes (water hyacinth) the pH remains slightly moderately acidic as shown in Table 1.0. It could be suggested here that in the soil mixture, the aluminum ion do no longer exist as Al³+ ion but it has been converted to aluminum hydroxide. This is in line with which says that exchangeable H+ that are released by base forming cations, move into the solution where it reacts with OH+ to form water [21].
Table 1.0: pH, Total Organic Carbon and Total Phosphorus Baseline
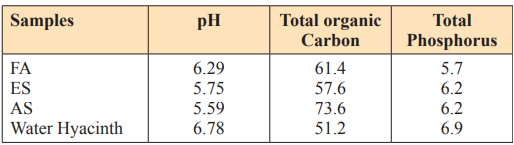
Table 1.1: pH of Amended Soil Samples
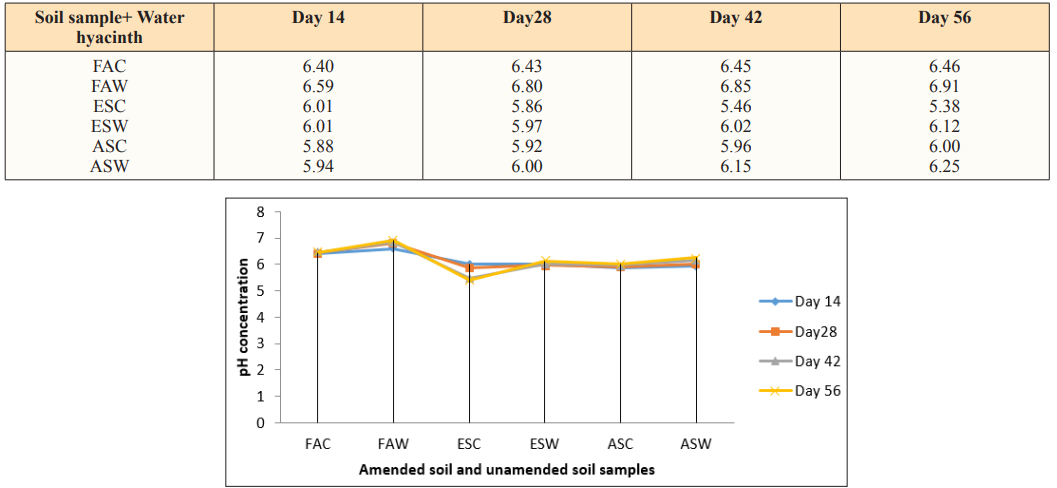
Table 2: Total Organic Carbon (TOC) in g/Kg at 40gram Loading Rate of Water Hyacinth
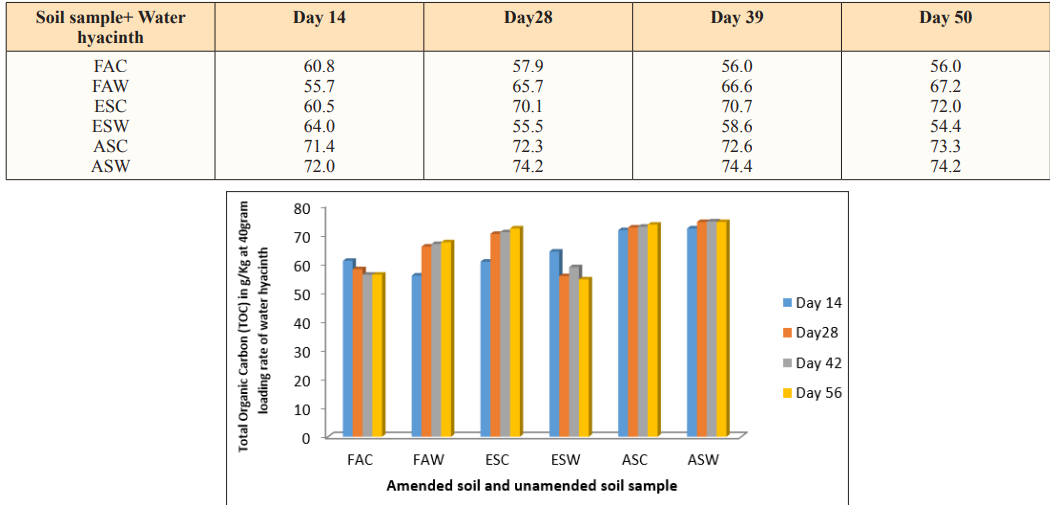
Figure 2: Total Organic Carbon (TOC) in g/Kg at 40gram Loading Rate of Water Hyacinth
Table 3: CO2 Evolved in g/Kg at 40gram Loading Rate of Water Hyacinth
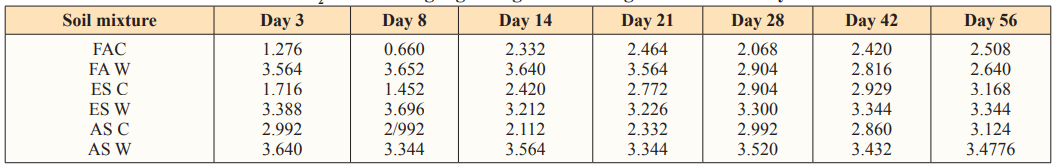
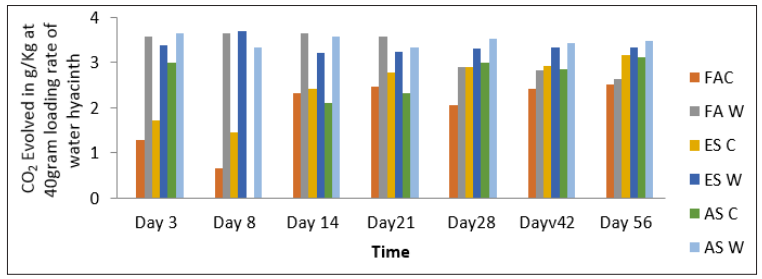
Figure 3: CO2 Evolved in g/Kg at 40gram Loading Rate of Water Hyacinth
Table 4: Cumulative CO2 evolved in g/Kg at 40gram Loading Rate of Water Hyacinth
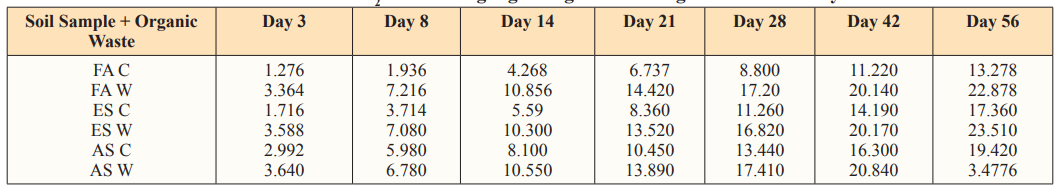
FAC = Fadama control
FAW =Fadama + water hyacinth
ESC= Enitsol control
ESW = Enitsol + water hyacinth
ASC = Acid Sulphate control
ASW = Acid Sulphate + water hyacinth
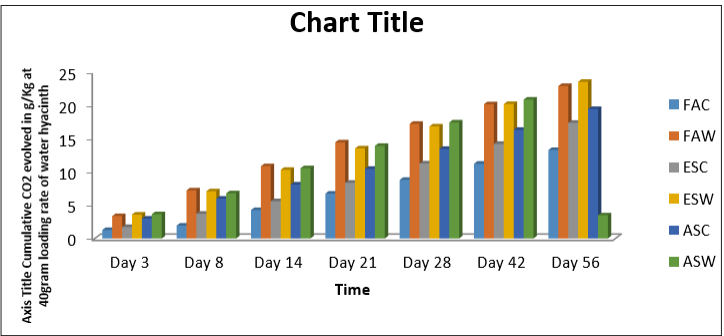
Figure 4: Cumulative CO2 evolved in g/Kg at 40gram Loading Rate of Water Hyacinth
Reaction Rates
The reaction rates of carbon mineralization in the control soils are higher than the amended soil in the first three days. This is attributed to a rapid decomposition of dead microbial biomass. After the initial flush, the decomposition rate of soil organic matter becomes lower. The initial flush was described by a first order kinetics as shown in Table 5. Independent of time, the organic matter remained in the soil, mineralization occurs in two distinct phases. The first rapid phase which corresponds to decomposition of the most labile produced by the microorganisms and second phase, slower phase between 14 days and 56 during which the most resistant organic product started to mineralize [5]. Data of the CO2 evolved fitted into the first order equation (log A =log A?- K+/2303) indicated that the reaction followed a first order equation.
Table 5: Reaction rates (K) Estimated according to first order equation [lo g (A) =log (Ao) - kt/2.303] at 40g Loading Rate of Water Hyacinth
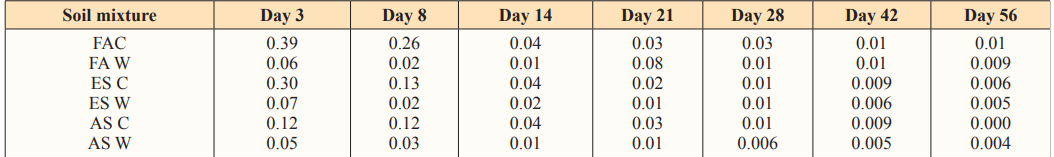
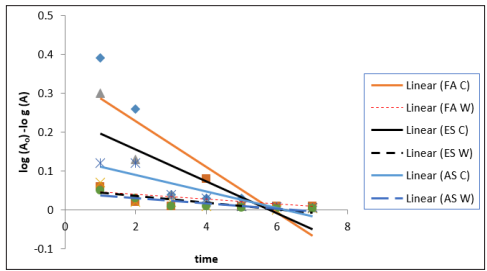
Figure 5: Reaction rates (K) Estimated According to First Order Equation [log (A) =log (Ao) - kt/2.303] at 40g Loading Rate of Water Hyacinth
Total and Available Phosphorus in Water Hyacinth Amended Soil and Unamended (Control) Soils
The total phosphorus in control soil and the amended soil tend to increase with time but with decrease in the control soil for the three soil samples than the amended soil. This could be attributed to the total phosphorus present in water hyacinth as seen in table 1.1 above added to the soil.
The available phosphorus increases with time in Fadama control soil than the amended soil due to the pH of Fadama soil that is at Goldilocks’ zone of its acidity (6.2) as seen in table 1.1. This is in line with that says soil at pH 6.2 yield to phosphorus availability [22,23]. The decrease in the available phosphorus in Fadama amended soil is attributed to the total phosphorus pH 6.78 which tend to neutralized the pH of the amended Soil. Enitsol soil and acid sulpate soil with pH 5.75 and 5.59 respectively yielded better amount of available phosphorus with time compared to their control due to the presence of water hyacinth with pH 6.78 bringing the soil mixture pH to average of 6.25 and 6.24 respectively.
Table 6: Total Phosphorus in g/kg at 40g Loading Rate of Water Hyacinth
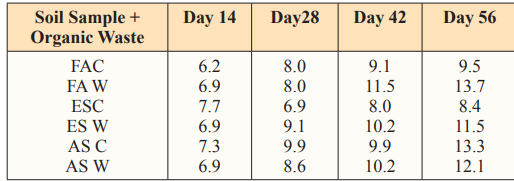
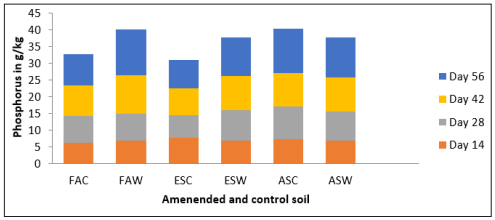
Figure 6: Total Phosphorus in g/kg at 40g Loading Rate of Water Hyacinth
Table 7: Available Phosphorus at 40gram loading rate of water hyacinth
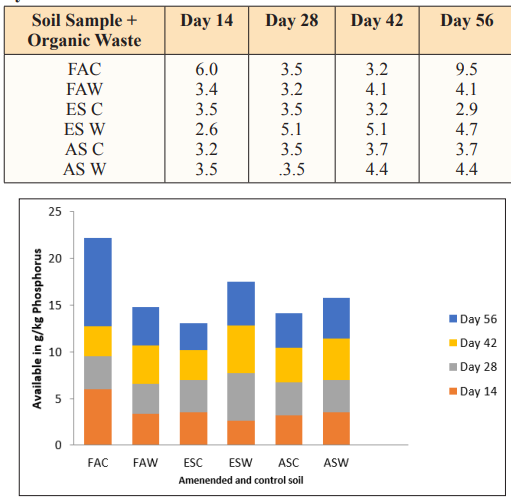
Figure 7: Available Phosphorus in g/kg at 40g Loading Rate of Water Hyacinth
Conclusion and Recommendation
In agreement with the authors, CO2 emission acted as an indicator of soil microorganism activities. The emission of CO2 was very similar in the three soil samples [20,24,25].
In the three amended soil, it was observed that the organic waste yielded good mineralization values when compared with the control.
In terms of total and available Phosphorus mineralization, increase in mineralization depends on the pH and the total organic matter released by the amended soil. In a nut shell, water hyacinth will serves as good organic manure when incorporated into Enitsol soil and acid sulphate soil. In a summary, the pH of the wetland soil needed to be considered when applying organic manure made of water hyacinth and if possible liming is recommended where the soil are found to be acidic or neutral to obtain a maximum yield of some vegetable crops such as garden egg, okra, cucumber, watermelon as well as maize and potatoes when planted in this wetland soil.
References
1.Hinsinger P (2001) Bioavailabilty of soil iorganic phosphorus in the Rizosphere as affected root-indused chemical changes: a review. Plant and Soil 273: 173-195.
2.Shen J, Lixing Yuan, Junling Zhang, Haigang Li, Zhaohai Bai, et al. (2011) Phosphorus dynamics: From soil to plant. Plant physiology 156: 997-1005.
3.Zielke RC, Christenson DR (1986) Organic Carbon and Nitrogen Changes in Soil under selected cropping system. Soil Science Society American Journal 50: 365-367.
4.Levi Minzi R, Riffald R, Saviozzi A (1990) Carbon and Phosphorus Mineralization in Soil Amended with different Organic material. Agricultural Ecosystem and Environment. 31: 325-335.
5.Nannipieri P (1990) Ecological Significance of the Biological activity in Soil, In J.M.Bolling and G.Stozky (Eds) Soil Biochemistry. Mareel Dekker Inc. New York 6: 293-354.
6.Jing Z, Ruirui Chen, Shiping Wei, Youzhi Feng, Jiabao Zhang, et al. (2017) Response and Feedback of carbon to Mineralization to phosphorus Availability driven by soil microorganisms. Soil Biol. Biochem 105: 111-120.
7.Chen J, Jasmin Seven, Thomas Zilla, Michaela A Dippold, Evgenia Blagodatskaya, et al. (2019) Microbial C: N: P stoichiometry and turnover on n Nutrient Availability in Soil: A14C,15N and 33P triple Labeling study. Soil Biochem 131: 206-216.
8.Uka UN, Chukwuka KS, Daddy F (2007) Effects of water Hyacinth (Eichhornia Crassipe) infestation on zooplankton populations in Awba Reservoir, Ibadan, South West Nigeria. 21st Annual Conference of the Fisheries Society of Nigeria (FISON) 99-105.
9.Agulo P, Amanda L Esperance, Elizabeth Mbau, Phillip Palmer, Asmita Patel, et al. (2007) Attracting Investment to Kisumu: opportunity and challenges. PDF Columbia University 1-25.
10.Doughty R, Turner W, Warnock M (2019) Unnatural Texas? The invasive Species Dilemma. Texas A&M University press 54-55.
11. Idachaba A (2015) How I turned a deadly plant into a thriving business. TED Women https://www.ted.com/talks.
12.Shaw-Hua Y, Wei S, Jun-Yao G (2016) Advances in management and utilization of invasive Water Hyacinth (Eichhornia Crassipe) in aquatic Ecosystem- A reviewer critical review in Biotechnology. Taylor and Francis 37: 218-228.
13.Wolverton BC, McDonald RC (1981) Energy from vascular plant wastewater treatment System-Eichhornia Crassipe, Duckweed (Spirodela sp, lemna sp.), Pennywort (Hydrocotyle ranunculoides), Pueraria Lobata, biomass harvested for fuel production. Economic Botany 35: 224-232.
14.Chea YM, Tabatabai MA (1986) Mineralization of Nitrogen in soils amended with Organic Wastes. Journal of Environmental Quality 15: 195-198.
15.Walkley A, Black CA (1934) Estimation of Soil Organic Carbon by the chromic acid titration method. Soil Science 37: 29-38.
16.Min ST, Han JS, Shin EW, Park JK (2004) Improvement of cadmium ion removal by base treatment of Jumper Fibre. Water Res 38: 1289-1295.
17.Pascual JA, Hernandez T, Garcia C (1978) Carbon Mineralization in arid Soil amended with Organic Wastes of varying degree of stability. Communication in Soil Science and Plant Analysis 29: 835-849.
18.Ademoroti CMA (1996) Standard Methods for Water Effluents Analysis. Foludex Press Ltd, Ibadan 70-77.
19.Isirimah NO (1987) An inventory of some chemical properties of selected surface soils of River state of Nigeria. In proceeding of 15th Annual conferences of Soil Science Association of Nigeria Kaduna 217-233.
20.Odu CTI, Nwoboshi LC, Esuruoso OF (1985) Environmental study (soil and vegetation) of the Nigerian Agip Oil Company Operation areas. Proceeding of the international seminar on the petroleum Industry and the Nigeria Environments Port Harcourt 274-283.
21.Brady NC (1974) The Nature and properties of soil. 8 edition, Macmillan publishing New York 143-144.
22.Margenot AJ, Parikh SJ, Rolf Sommer (2018) Soil phosphate activities across liming gradient under long term Management in Kenya. Soil Biology & Biochemistry 82: 850-861.
23.Mallarino AP, Sawyer JE, Barnhart SK (2013) Crop Nutrient and Limestone recommendation in Iowa PM 1688.
24.Saviozzi S, Levi MR, Riffaldi R (1993) How Organic Matter sources affect Cadmium movement in Soil. Biocycle 24: 29-31.
25.Garcia C, Costa F, Hernández T (1994) Microbial activity in Soil under Mediterranean Environment Conditions. Soil Biochem 26: 1185-1191.

