An Electrochemical Biomolecular Sensors Based on Polymeric Ion Conducting Nanopores for Medical Diagnostics
© 2023 Wolfgang Ensinger, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In a biomimetic approach, the functionality of biological nanopores in living cells has been copied in order to create polymeric nanochannels. In analogy to the antitype in nature, the nanochannels are able to respond to the presence of a certain biomolecule to be detected in a way that an electrolyte ion current in an electrochemical set-up is influenced. By measuring this trans-nanochannel ion current, the molecule to be analyzed can be quantitatively determined. As an example, the fabrication and functionality of such a nanochannel-sensor for the biomolecule histamine is demonstrated and explained.
Introduction
Sensing and quantitative analysis of biologically relevant molecules is an important aspect of medical diagnostics and also environmental control, such as for groundwater analysis. Here, a biomimetic approach is presented, based on nanopores in living cells [1,2]. The latter regulate the flow of different species, both ions and small molecules, through the cell membrane into and out of the cells. This transport is regulated by different stimuli. One such stimulus can be a certain biomolecule, the signal molecule or ligand. When this biomolecule in present, the ligand is attached to a certain part of the nanopore, the receptor. This bioconjugation reaction works with the key-lock-principle: ligand and receptor need to fit to each other. The nanopore opens and lets species, such as ions, pass, see Figure 1. This principle can be used for sensing. The transport of ions of an electrolyte through the nanopores can be monitored. When the ion flow is measured, meaning that the nanopore is in the open position, the external stimulus must be present. Next to this qualitative measurement, the sensing can also be carried out quantitatively, if the amount of ion flow depends on the amount of the biomolecule.
This is a very sensitive and potentially also selective quantification principle. It works well with biological nanopores. The drawback with these, however, is that they are carried by the fragile lipid bilayer membrane of the cell. This impairs storage time and usability. Biological nanopores are not suitable for various technical applications and need, therefore, to be replaced by solid state nanopores, in the present case by polymer-based ones.
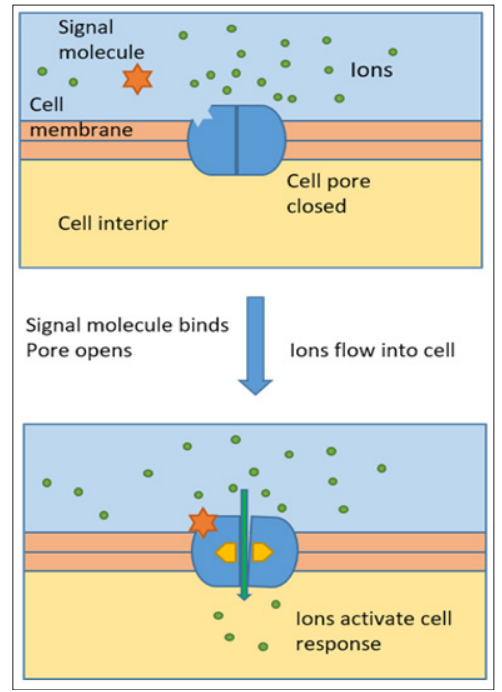
Figure 1: Schematic of function of Nanopore in living cell: the signal molecule activates the pore to open, ion flow is established
Experimental
For fabricating the nanochannels in polymer, Polyethylene terephthalate (PET) foils of 12 µm thickness were irradiated at the linear accelerator UNILAC (GSI Helmholtz-Center of Heavy Ion Research, Darmstadt, Germany) with a single gold ion with a kinetic energy of 2.2 GeV. The highly energetic ion crosses the polymer foil completely along a straight line, the ion track. It transfers a part of its kinetic energy into the electronic system, typically several 1000 eV per nm length of the ion trajectory. As a consequence, chemical bonds within the polymer are broken. Small volatile molecular fragments are cut out of the polymer backbone. The ion damage track is a cylindrical zone of several nm diameter, with a lower density and reduced chemical stability. The ion track is etched up into a conical nanopore, here called nanochannel, due to its large aspect ratio. For an asymmetric etching, the polymer foil was placed between two separated compartments of a glass cell, one containing 9 molar Sodium Hydroxide (NaOH) as etchant, the other one 1 molar Formic Acid (HCOOH) as an etch stop. This neutralized the etchant after the breakthrough. The process is electrochemically controlled [3]. The large aperture of the conical nanochannel is typically in the region of several hundred nm in diameter, the small at the etch stop side a few to some ten nm. Figure 2 shows a Scanning Electron Micrograph of the two apertures in the polymer foil.
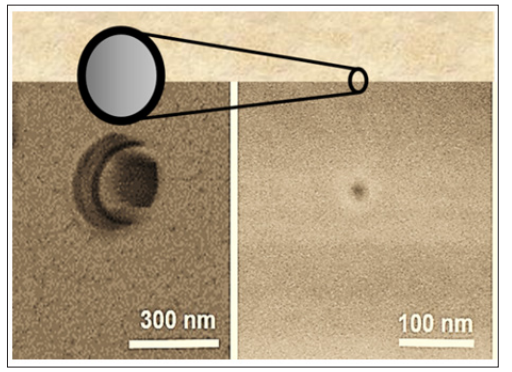
Figure 2: Scanning electron micrograph of apertures of a conical nanochannel in PET foil
The etching takes place via breaking the ester bonds of the polyester PET, leaving an internal nanochannel surface that contains carboxylate groups (-COOH). Those were used to functionalize the nanochannel by means of covalent coupling via a reaction with (N-(3-dimethyl-aminopropyl)-N'-ethylcarbodiimide- hydrochloride (EDC) and Pentafluorophenol (PNP), with concentrations of 100 mM and 200 mM, resp., in ethanol for 1 hour at room temperature. Next, a reaction with 25 mM of Bis(carboxymethyl)-L-lysine hydrate (BCML) in an 8:2 ethanol/ water mixture was carried out, coupling this molecule to the nanochannel walls by its amino group. The reaction product is shown in Figure 3.
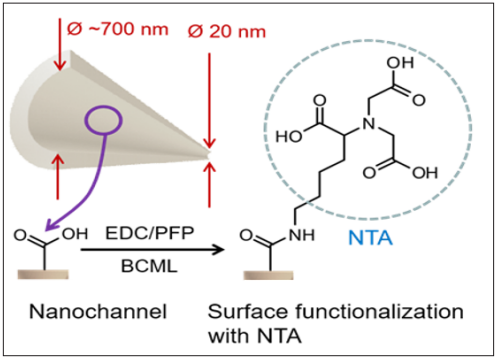
Figure 3: Immobilization of Nitrilotriacetic acid (NTA) on nanochannel surface via covalent coupling to carboxylate groups at the nanochannel surface.
The BCML molecule contains Nitrilotriacetic (NTA) acid moieties that can act as chelating agent. It creates a coordination compound with various transition metal ions. Thus, by adding an alkaline aqueous solution of 100 mM Nickelsulfate (NiSO ), an NTA-Ni2+ chelate was generated inside the nanochannel, see Figure 4.
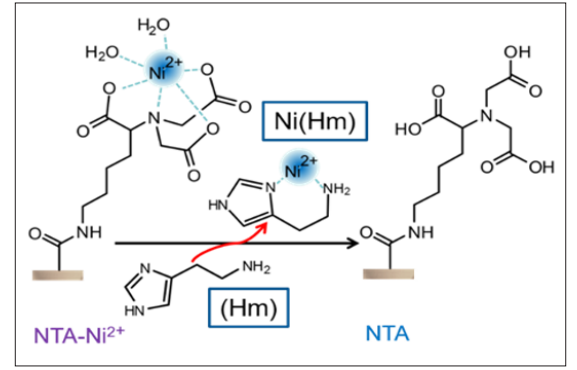
Figure 4: Formation of an NTA-Ni2+ complex; sensor reaction: binding of the nickel ions by Histamine (Hm), setting free neutral NTA on the nanochannel surface
For the sensor, an electrochemical 2-compartment cell was used, with the polymer foil with the nanochannel being the separator. A schematic is shown in Figure 5 and a photograph in Figure 6. The cell consists of the inert polymer Polytetrafluoroethylene (PFTE), with the two compartments pressed together by a screw for sufficient sealing, and inserted into a metallic housing for shielding.
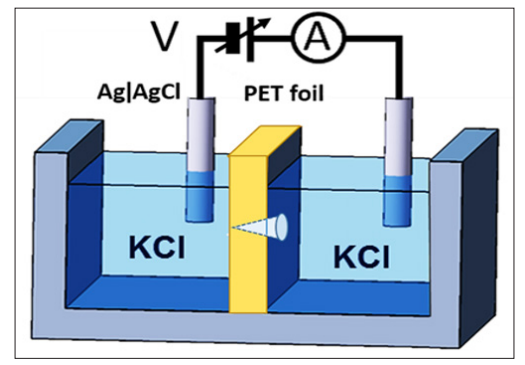
Figure 5: Schematic of electrochemical two compartment cell for ion current measurements through the conical nanochannel in the PET foil. The electrolyte is an aqueous solution of Potassium Chloride (KCl)
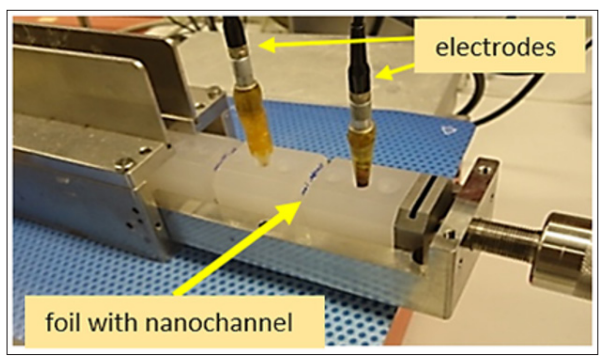
Figure 6: Electrochemical cell with 2 PTFE compartments, separated by the PET foil with nanochannel, and 2 AgïAgCl electrodes
Aqueous 0.1 M KCl solution was used as electrolyte. The flow of the ions, i.e. the ion current I, is quantitatively measured as a function of the voltage V that is applied between the two standard reference Ag/AgCl electrodes. The voltage/current meter was a Picoammeter 6487 (Keithley Instruments). The voltage V was ramped up from -1 to +1V, in steps of 100 mV, and the transnanochannel ion current I was measured.
Results and Discussion Histamine Sensing
When an aqueous salt-containing electrolyte is inserted into the cell and a voltage is applied, an ion current, consisting of K+ and Cl- depending on the polarity, flows between the electrodes through the nanochannel. Upon increasing the voltage, the ion current flow increases, as shown in Figure 7. The increase is almost linear. The ion current depends on the ion concentration, the diameter of the nanopore and the surface charge of the nanochannel walls. The latter is defined by the NTA-Ni2+ moieties on the surface.
When only 1 nanoMole of Histamine (Hm), the well-known neurotransmitter, is added, the histamine removes a portion of the Nickel ions from the NTA surface due to a stronger binding force [4]. A part of the surface remains with uncharged NTA. With the surface now having a lower amount of positive charges, the K+ ion transfer through the nanochannel, hindered by the repulsive forces of the NTA-Ni2+ complex, increases. This larger ion current in presence of Hm is significant. With a molar mass of 111 g/ mole, a 1nM solution corresponds to ca. 0.1 µg Histamine per liter water. i.e. a very small quantity.
When the Histamine concentration is further increased, the current rises further. This increase goes on over 6 orders of magnitude, see Figure 8. From the ion current and the nanochannel dimensions, the conductivity S of the nanochannel, given in nS, can be calculated. Figure 7 shows that the conductivity is an almost linear or, at least, a monotone function of the histamine concentration, over several orders of magnitude. At higher concentrations (> 10-4), the curve flattens, as the saturation level is reached.
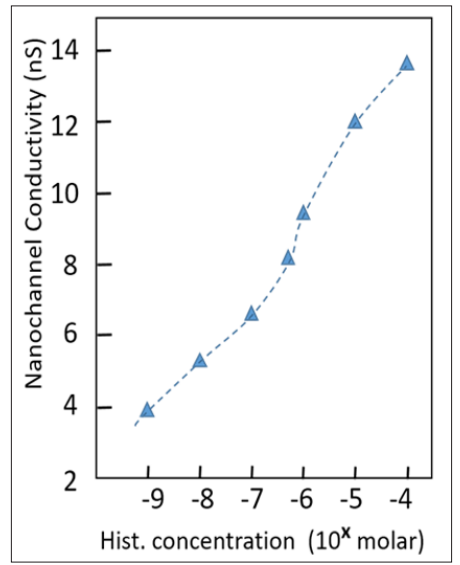
Figure 7: Current/voltage curves of different concentrations (in NanoMole) of Histamin.
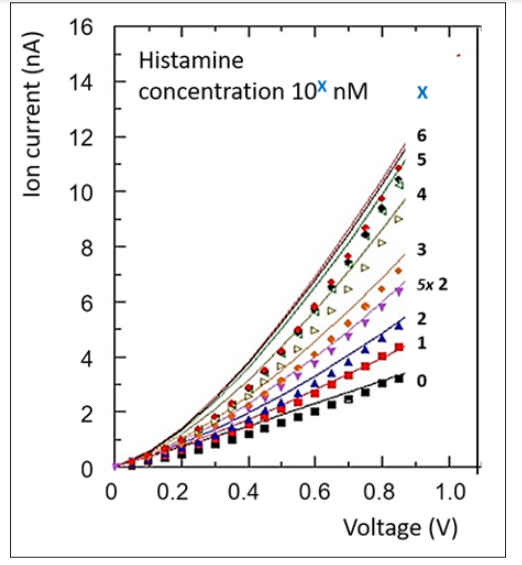
Figure 8: Electrical conductivity, derived from current/voltage curves, as a function of Histamine concentration in the solution. The dashed line is a guide to the eye only.
Other neurotransmitters, namely glycine, serotonin, gamma- aminobutyric acid (GABA}, and dopamine (DA), do not react with the nanochannel in this way [5]. However, other chemicals, that bind Nickel ions, would. Therefore, such a sensor, as sensitive as it is, has always to be investigated for its selectivity, depending on the analyte and its molecular environment.
Further examples of biomolecular sensing are Saccharide/ glycoprotein, phosphoprotein and specific protein detection [6-8].
From Lab Set-up to Lab-on-Chip
For technical applications, it is too time-consuming and fault- prone to mount the nanochannel-containing foil; also its storage is problematic. Therefore, a compact chip would be a better solution. For this purpose, the nanochannel carrying polymer foil has been incorporated in a microfluidic electronics device. Figure 9 shows a schematic cross-section. Layers of plastic sheets with differently shaped slots are used. They are glued onto a substrate, sequentially, thus forming the electrochemical two- compartment cell. The polymer foil with nanochannels separates two microfluidic channels, each one with a gold layer that serves as an electrode.
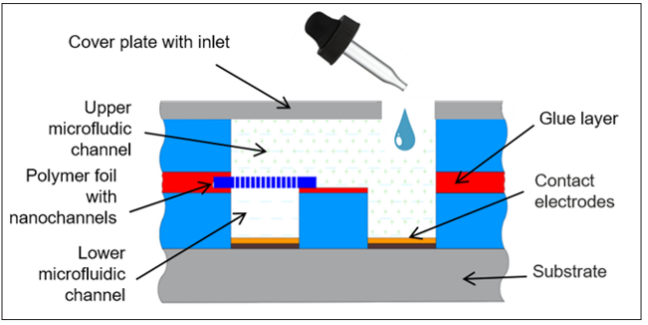
Figure 9: Schematic cross-section of microfluidic Lab-on-a- Chip
Figure 10 shows a photographs of the chip, with the micro channels and electrodes. Its dimensions are 2.3 x 1.4 cm2.
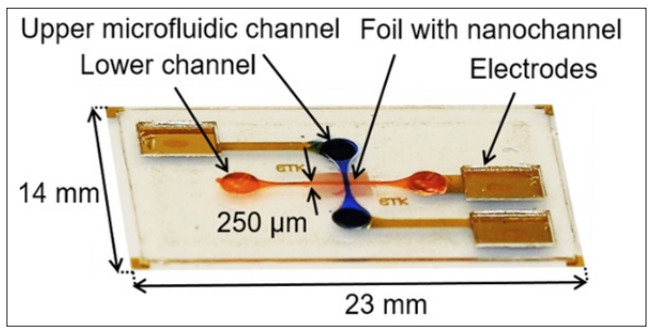
Figure 10: Photograph of Lab-on-a-Chip Device
This functionality of the chip, in combination with a voltage/ current meter, has been demonstrated with Histamine sensing with the presented chemical functionalization of the nanochannels. It turned out that it worked, but with a lower sensitivity. This is probably due to the observed higher noise background.
Apart from sensitivity, another issue is selectivity. When a single sensor is not specific enough, an array needs to be used. This would be one of the next steps in development. The multicell set- up, as schematically shown in Figure 11, would contain several individual sensors, for a given solution to be analyzed. Thus, cross sensitivities of interfering species could be ruled out. The sensor array would give different patterns for certain combinations of concentrations of the different molecules. This would, however, require a careful calibration.
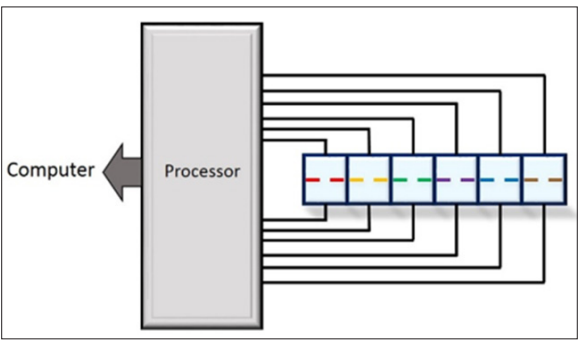
Figure 11: Schematic of Multicell Set-up with an Array of Sensors
Conclusion
It has been demonstrated that a chemically functionalized polymeric nanochannel can be used as the core of an electrochemical sensing device for biomolecules in aqueous solution. For the further development into clinical/industrial application, several issues need to be addressed. The influence of further components of the liquid to be analyzed has be investigated. For many case, a separation pre-step might be required, e.g. by using microfluidic chromatography. It has to be made sure that a certain selectivity is given. For this purpose, an array of individually functionalized nanochannels for interfering molecules could be used.
Another development, particularly into time-dependent studies, would be the use of a flow-through cell where the analyte solution is pumped through a cell that is equipped with a microfluidic by- pass channel. Thus, for instance, pharmacokinetic studies would be available.
Acknowledgment
The author gratefully acknowledges the fruitful collaboration with Drs. Mubarak Ali, Saima Nasir, Ivana Duznovic, Mario ElKhoury, and Prof. Helmut Schlaak †.
References
- H Bayley, PS Cremer (2001) Stochastic sensors inspired by Nature 413: 226-230.
- De La Escosura-Muniz, A Merkoci (2012) Nanochannels Preparation and Application in Biosensing. ACS Nano 6: 7556-7583.
- P Y Apel, Y E Korchev, Z Siwy, R Spohr, M Yoshida (2001) Diode-like single-ion track membrane prepared by electro- Nucl. Instrum. Methods Phys. Res. B 184: 337-346.
- S Nuutinen, P Panula (2010) Histamine in neurotransmission and brain Adv. Exp. Med. Biol 709: 95-107.
- M Ali, P Ramirez, I Duznovic, S Nasir, W Ensinger, et al. (2017) Label-free histamine detection with nanofluidic diodes through metal ion displacement mechanism. Colloids and Surfaces B: Biointerfaces 150: 201-208.
- Q H Nguyen, M Ali, R Neumann, W Ensinger (2012) Saccharide/glycoprotein recognition inside synthetic ion channels modified with boronic Sensors and Actuators B: Chemical 162: 216-222.
- S Nasir, M Ali, I Ahmed, C M Niemeyer, W Ensinger (2020) Phosphoprotein Detection with a Single Nanofluidic Diode Decorated with Zinc ChemPlus Chem 85: 587-594.
- Duznovic A Gräwe, W Weber, LK Müller, M Ali, W Ensinger, et al. (2021) Ultrasensitive and Selective Protein Recognition with Nanobody-Functionalized Synthetic Small 17: 2101066.

