Human Leukocyte Antigen Mismatch is Associated with Grade 3 Primary Graft Dysfunction at 72 Hours Following Bilateral Sequential Lung Transplantation: A Single-Center, Retrospective Cohort Study
© 2021 Tjorvi E Perry, Roy Kiberenge, Megan Olejniczak, Scott Jackson, Stephen Richardson et, al, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The role of donor-recipient human leukocyte antigen (HLA) mismatch as a risk factor for developing primary graft dysfunction (PGD) after lung transplantation is not well understood. We describe a novel association between increased donor-recipient HLA mismatch and grade 3 PGD after bilateral lung transplantation.
Methods: We retrospectively evaluated donor and recipient demographic data, co-morbidities, intraoperative interventions and outcomes in 99 consecutive adult patients undergoing primary bilateral lung transplantation. The primary outcome of this study was grade 3 PGD at 72 hours. Secondary outcomes included intensive care and hospital lengths of stay and mortality.
Results: Eighteen patients (18%) met criteria for grade 3 PGD at 72 hours postoperatively. More non-Caucasian recipients (27.8% vs. 7.4%, p=0.026), and more patients with interstitial lung disease (72.2% vs 43.2%, p=0.031) developed grade 3 PGD. The use of inhaled epoprostenol (OR 4.38, 95% CI: 1.02-20.16, p=0.048), increased HLA mismatches (OR 2.85, 95% CI: 1.31-7.45, p=0.017) and the use of each 250mL unit of PRBCs during the intraoperative period (OR 0.77, 95% CI: 0.58-0.97, p=0.048) were independently associated with grade 3 PGD. Patients diagnosed with grade 3 PGD spent significantly longer time in the intensive care unit (22 days [6;74 days] vs. 7 days [2;83 days], p=<0.001) and hospital (30.5 days [10;83 days] vs. 18 days [3;97 days], p=0.012), and survival was significantly worse for those with PGD3 at 72 hours (log-rank p=0.009).
Conclusion: Our data indicate, for the first time, that HLA donor-recipient mismatch is an independent risk factor for developing grade 3 PGD at 72 hours after bilateral lung transplantation.
Introduction
Lung transplantation remains the definitive therapy for
patients with end-stage lung disease and respiratory failure.
Despite advances in surgical techniques, patient selection, and
postoperative management strategies, mortality rates following
lung transplantation remain as high as 15% at one year and
50% at five years after transplant [1,2]. Severe primary graft
dysfunction (PGD) after lung transplantation has an incidence
ranging from 10-50%, and has been identified as an independently
contributing risk factor for postoperative mortality [1-5]. In a
recent 10-center study with 1255 patients having undergone lung
transplantation, the authors describe an array of perioperative
variables independently associated with developing severe
postoperative PGD including any history of donor smoking,
increased inspiratory oxygen concentration (FIO2) during lung
reperfusion, use of cardiopulmonary bypass, high recipient body
mass index (BMI), and high mean recipient pulmonary artery
pressure [6]. Although immunologic injury is thought to be
another potential contributor to severe PGD and HLA mismatch
has been associated with decreased long-term survival after lung
transplantation, the exact role of donor-recipient human leukocyte
antigen (HLA) mismatch in development of severe PGD is not
well-defined [7]. While one recent study suggests an increased
incidence of PGD grade 2-3 at 48 hours in patients with early
donor specific antibody (DSA) development, a previous study
found that PGD was associated with the subsequent development
of anti-HLA class II but not anti-HLA class I DSA [8]. While
these previous studies examined the relationship between DSA
development and PGD, no previously published study has reported
the relationship between HLA mismatch and severe PGD [9].
Herein, we describe donor and recipient risk factors, specifically
including donor-recipient HLA mismatch, that are associated with
developing grade 3 PGD in the first 72 postoperative hours in
adults undergoing bilateral lung transplantation at a single-center
quaternary care academic medical center.
Methods
In this single center, retrospective cohort study evaluating all primary
bilateral lung transplantation in adults between January 1, 2016 and
December 31, 2018, donor and recipient demographic data, donor
and recipient co-morbidities, intraoperative interventions including
incision type, type of intraoperative mechanical support, laboratory
results, intraoperative mechanical ventilation strategy, fluid and blood
product administration, and outcomes were collected from several
hospital registries as well as our electronic medical record (Epic
Systems Corporation, Verona, Wisconsin) and United Network for
Organ Sharing (UNOS). Our study was approved by the University
of Minnesota institutional review board (#1006M83333), which
waived the need for consent from individual patients. Patients who
had opted out of being included in research studies were excluded.
Donor lungs were preserved with either cold static storage or warm ex vivo lung perfusion (OCSTM Trans Medics Inc, Andover, MA). Intraoperative management for each case including the use of double lumen or single lumen endotracheal tube, volume resuscitation, blood product administration and mechanical ventilatory strategy was at the discretion of the surgical and anesthesia team. All bilateral lung transplantations were carried out using either CPB or VA ECMO through either a clamshell incision or sternotomy, depending on recipient anatomy.
The primary outcome of this study was grade 3 PGD at 72 hours postoperatively based on the 2005 International Society for Heart and Lung Transplantation (ISHLT) PGD working group definition [10,11]. To meet this definition, we calculated a partial pressure of arterial oxygen divided by the fraction of inspired oxygen (P/F ratio) 72 hours postoperatively, and at the same time reviewed the chest radiograph for diffuse parenchymal infiltrates. Secondary outcomes included hospital and ICU lengths of stay and mortality.
Variables of interest were compared between those with and without grade 3 PGD at 72 hours. Categorical variables are displayed as n (%) and compared with Chi-square or Fisher tests. Continuous variables are displayed as median [range] and compared using a Wilcoxon non-parametric test. A logistic regression model was performed for the outcome of grade 3 PGD at 72 hours. All analysis was performed in R ver. 3.6.1.
Results
A total of 105 patients underwent primary bilateral lung
transplantation between January 1, 2016 and December 31,
2018. Of these, six patients declined participation in our research
database. Of the 99 remaining patients, eighteen patients (18%)
met criteria for the primary outcome of grade 3 PGD at 72 hours
postoperatively. On univariate analysis, there was no difference
in donor or recipient age or gender, or recipient body mass index
(BMI) between patients who developed grade 3 PGD at 72 hours
and those that did not (Table 1). There was no difference in mean
recipient pulmonary artery pressure, donor smoking history or
donor alcohol use. There was no difference in the use of either CPB
or VA ECMO, graft ischemic time, or the use of the Transmedics
Lung OCS System (OCSTM TransMedics Inc, Andover, MA).
Significantly more non-Caucasian recipients (27.8% vs. 7.4%, p=0.026), and proportionately more patients with interstitial lung disease (72.2% vs 43.2%, p=0.031) developed grade 3 PGD at 72 hours, postoperatively. Patients with a higher degree of HLA mismatch were more likely to develop grade 3 PGD at 72 hours postoperatively (5.0 [4.0;6.0] vs. 4.0 [1.0-6.0], p=0.024). Patients who developed grade 3 PGD at 72 hours postoperatively were also more likely to have had inhaled epoprostenol initiated intraoperatively (55.6% vs 21.0%, p=0.007), and to have received intraoperative allogeneic packed red blood cells (PRBC) (475mL [0;3,300] vs 0mL [0;8,700], p=0.032), fresh frozen plasma (FFP) ) (611mL [0;2,947] vs 292mL [0;5,880], p=0.048), platelets (528mL [0;2,072] vs 204mL [0;2,554], p=0.037) or cryoprecipitate (158mL [0;836] vs 0mL [0;620], p=0.017).
| No PGD (n = 81) | PGD (n = 18) | p value | |
|---|---|---|---|
| Recipient Gender | 0.463 | ||
| Female | 42 (51.9%) | 7 (38.9%) | |
| Male | 39 (48.1%) | 11 (61.1%) | |
| Donor Gender | 0.34 | ||
| Female | 28 (34.6%) | 9 (50%) | |
| Male | 53 (65.4%) | 9 (50%) | |
| Recipient Age (yrs) | 58.1 (24.1-69.3) | 56.7 (24.3-69.3) | 0.737 |
| Donor Age (yrs) | 32.7 (14.3-68.1) | 34.6 (16.2-57.9) | 0.792 |
| Incision Type | 0.979 | ||
| Clamshell | 23 (29.6%) | 6 (33.3%) | |
| Median Sternotomy | 57 (70.4%) | 12 (66.7%) | |
| Recipient Race | 0.026 | ||
| Caucasian | 75 (92.6%) | 13 (72.2%) | |
| Non-Caucasian | 6 (7.4%) | 5 (27.8%) | |
| Donor Race | 0.291 | ||
| Caucasian | 66 (81.5%) | 17 (94.4%) | |
| Non-Caucasian | 15 (18.5%) | 1 (5.56%) | |
| Etiology of Respiratory Failure | 0.031 | ||
| Cystic Fibrosis | 16 (19.8%) | 3 (16.7%) | |
| COPD | 28 (34.5%) | 1 (5.56%) | |
| ILD / IPF | 35 (43.2%) | 13 (72.2%) | |
| Other | 2 (2.4%) | 1 (5.56%) | |
| Mechanical Support | 0.297 | ||
| CPB | 69 (85.2%) | 13 (72.2%) | |
| VA ECMO | 12 (14.8%) | 5 (27.8%) | |
| Recipient BMI | 24.8 (17.2-31.6) | 26.1 (17.9-30.9) | 0.253 |
| Donor Smoking History | >0.999 | ||
| Yes | 11 (13.6%) | 2 (11.1%) | <? |
| No | 70 (86.4%) | 16 (88.9%) | |
| Donor Heavy Alcohol Use | 0.347 | ||
| Yes | 20 (24.7%) | 2 (11.1%) | |
| No | 61 (75.3%) | 16 (88.9%) | |
| Post-perfusion PaCO2 (mmHg) | 43 (31-64) | 41 (32-63) | 0.204 |
| Mean PAP (mmHg) | 26 (11-80) | 28.5 (16-44) | 0.7 |
| Inhaled Pulmonary Vasodilator | 0.007 | ||
| Yes | 17 (21%) | 10 (55.6%) | |
| No | 64 (79%) | 8 (44.4%) | |
| Graft Ischemic Time (min) | 343 (204-851) | 364 (191-843) | 0.577 |
| Use of Transmedics OCSTM | >0.999 | ||
| Yes | 8 (9.88%) | 2 (11.1%) | |
| No | 73 (90.1%) | 16 (88.9%) | |
| HLA Matches (numbe r, out of 6) | 4 (1-6) | 5 (4-6) | 0.024 |
| Intraoperative IVF (ml) | 2031 (400-4700) | 1800 (507-3800) | 0.576 |
| Intraoperative colloid (ml) | 0 (0-3000) | 125 (0-1700) | 0.096 |
| Intraoperative RBC (ml) | 0 (0-8700) | 475 (0-3300) | 0.032 |
| Intraoperative FFP (ml) | 292 (0-5880) | 611 (0-2947) | 0.048 |
| Intraoperative platelets (ml) | 204 (0-2554) | 528 (0-2072) | 0.037 |
| Intraoperative cryoprecipitate (ml) | 0 (0-620) 158 | (0-836) | 0.017 |
| RBC volume >1L | 0.041 | ||
| Yes | 12 (14.8%) | 7 (38.9%) | |
| No | 69 (85.2%) | 11 (61.1%) | |
| Initial postoperative FiO2 | 80 (40-100) | 95 (50-100) | 0.104 |
Abbreviations: PGD, primary graft dysfunction, ILD, interstitial lung disease, IPF, Interstitial pulmonary fibrosis, VA ECMO, venous arterial extracorporeal membrane oxygenation, CPB, cardiopulmonary bypass, BMI, Body mass index, PAP, pulmonary artery pressure, OCS, organ care system, HLA, human leukocyte antigen
The use of inhaled epoprostenol (OR 4.38, 95% CI: 1.02-20.16, p=0.048), increased HLA mismatches (OR 2.85, 95% CI: 1.31- 7.45, p=0.017) and the use of each 250mL unit of PRBCs during the intraoperative period (OR 0.77, 95% CI: 0.58-0.97, p=0.048) were independently associated with grade 3 PGD at 72 hours postoperatively (Table 2 and Figure 2). Patients diagnosed with grade 3 PGD at 72 hours spent significantly longer time in the intensive care unit (22 days [6;74 days] vs. 7 days [2;83 days], p=<0.001) and hospital (30.5 days [10;83 days] vs. 18 days [3;97 days], p=0.012) (Table 3). Post-transplant survival was significantly worse for those with grade 3 PGD at 72 hours (log-rank p=0.009, Figure 1).
| Adjusted Odds Ratio | 95% CI | p value | |
|---|---|---|---|
| Independent Risk Factors | 4.38 | 1.02-20.16 | 0.048 |
| Inhaled Pulmonary Vasodilator | 2.85 | 1.31-7.45 | 0.017 |
| RBCs per 250cc | 0.77 | 0.58-0.97 | 0.048 |
Abbreviations: PGD, primary graft dysfunction, HLA, human leukocyte antigen, RBCs, red blood cells
| No PGD (n = 81) | PGD (n = 18) | p value | |
|---|---|---|---|
| Clinical Outcomes | |||
| ICU LOS (days) | 7 [6;74] | 22 [2;83] | <0.001 |
| HLOS (days) | 18 [3;97] | 31 [10;82] | 0.012 |
Abbreviations: PGD, primary graft dysfunction, ICU LOS, intensive care length of stay, HLOS, hospital length of stay
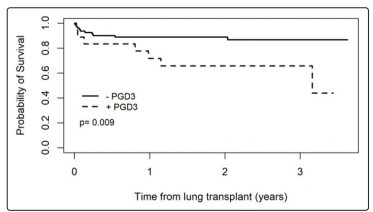
Figure 1: Morality associated with and without developing grade 3 primary graft dysfunction at 72 hours
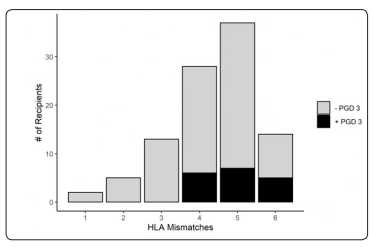
Figure 2: The incidence of grade 3 primary graft dysfunction stratified by HLA mismatch
Discussion
In this single-center retrospective analysis, we found an 18%
incidence of grade 3 PGD at 72 hours postoperatively after
bilateral sequential lung transplantation in adults. On univariate
analysis, non-Caucasian recipient, primary diagnosis of interstitial
lung disease, the use of intraoperative epoprostenol, the need for
transfusion with RBCs, FFP, platelets and cryoprecipitate, and
donor-recipient HLA mismatch were associated with an increased
probability of grade 3 PGD at 72 hours postoperatively. On
multivariate analysis, grade 3 PGD at 72 hours was independently
associated with the use of inhaled, degree of HLA mismatch and
the use of PRBC in excess of one liter during the intraoperative
period. Our data also suggest a correlation between developing
grade 3 PGD at 72 hours postoperatively and increased intensive
care unit and hospital lengths of stay, and increase in long-term
mortality.
PGD remains the leading cause of early death after lung transplantation in adults with a 15-20% incidence rate at 48- or 72-hours post-transplant [5, 6, 12]. Shortly after the International Society for Heart and Lung Transplantation (ISHLT) working group published the classification system that we currently use to define PGD after lung transplantation, Whitson et al. reported a 17% grade 3 PGD incidence rate at 48 hours postoperatively in 402 consecutive adult patients who had undergone lung transplantation between 1992 and 2004 [4,10,11]. In this study, grade 3 PGD at 48 hours was associated with decreased overall BOS-free survival in this study. In a more recent prospective cohort analysis of 1255 adults undergoing lung transplantations across 10 centers in the United States, the investigators cite a 16.8% incidence of grade 3 PGD at 48 to 72 hours after transplantation, while Samano et al. reported a 15.4 % incidence of grade 3 PGD after 72 hours post-transplantation [6,13]. Despite our relatively small sample size, we corroborate that a 15-20% incidence of grade 3 PGD after 72 hours after lung transplantation has gone unchanged in the last decade.
From the time the ISHLT working group published their original definition in 2005, investigators have compiled a lengthy list of donor and recipient risk factors for PGD. Donor risk factors including smoking history, alcohol use, and lung trauma (contusions, fat emboli or pulmonary thromboembolic events) are associated with increased risk of PGD [4,6,12,14-17]. Recipient risk factors include obesity, and disease processes associated with either primary or secondary pulmonary hypertension [6,12,18- 20]. Investigators have also identified a number of perioperative risk factors including the use of cardiopulmonary bypass, large- volume intraoperative transfusion, and an inspired oxygen fraction of greater than 0.4 during lung reperfusion [6,21-23]. Data supporting the association between donor and recipient demographics including age, gender and ethnicity have been conflicting, as has prolonged ischemic time [6, 12, 18, 24-26]. In our cohort, we found similar risk factors associated with grade 3 PGD at 72 hours postoperatively including recipient race, primary diagnosis of interstitial lung disease, and the use of blood products including high volumes of RBCs. Blood product transfusion is a known risk factor for acute lung injury, and in the setting of lung transplantation, should be considered a modifiable risk factor. As such, we often consider factor and fibrinogen concentrates in lieu of allogeneic blood products when addressing non-surgical coagulopathy in our lung transplantation patients [27]. Although we did not find a difference in preoperative mean pulmonary artery pressures, as Whitson et al. described, we found that the use of inhaled epoprostenol was significantly higher in patients who developed grade 3 PGD at 72 hours [4]. Due to the retrospective nature of our study, it is unclear whether inhaled epoprostenol was initiated in order to address poor oxygenation or elevated pulmonary artery pressures. In our institution, inhaled epoprostenol is used for both purposes, and as such, inhaled epoprostenol could be a marker for pulmonary hypertension or an indicator of poor oxygenation after reperfusion.
Finally, we know that matching donor and recipient for HLA antigens significantly decreases the risk of organ rejection [28]. To avoid prolonged ischemic times, HLA typing of the donor is completed prior to removing the organs, a practical challenge for lung transplantation, and a reason HLA matching is rarely done for lung transplantation patients. Even if this were feasible, the extensive polymorphism of the HLA system would make an exact match (zero HLA mismatched donor-recipient) an extremely rare event [29]. The median number of HLA mismatches in lung transplantations on a scale of 0-6 has been shown to be 4 [30]. A number of studies have shown HLA mismatch (>3) to be a strong predictor of long-term graft survival and mortality [7, 31-33]. To the best of our knowledge, we are the first to describe HLA donor-recipient mismatch as an independent risk factor for developing grade 3 PGD at 72 hours postoperatively, even in our relatively small cohort. As of now, HLA classification is a non- modifiable risk factor. In light of our findings however, and due to the significant morbidity and mortality associated with PGD after lung transplantation, we would urge other centers to examine the association between HLA classification and the incidence of PGD in their lung transplantation population. As UNOS progresses toward an allocation system of continuous distribution, HLA mismatch might be incorporated as a risk factor [34].
The limitations of this single-center study include all biases associated with a retrospective design. Additionally, our sample size is small relative to similar studies. Our analysis however is based on data from very detailed and well-maintained institutional lung transplantation and UNOS databases.
Acknowledgements
We would like to acknowledge the significant contribution of
Maribet McCarty, Lead Health Information Data Analyst, in
helping us gather and compile perioperative data from EPIC.
References
1. Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal
NP, et al. (1998) Primary graft failure following lung
transplantation. Chest 114: 51-60.
2. Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C,
et al. (2005) Impact of primary graft failure on outcomes
following lung transplantation. Chest 127: 161-165.
3. Christie JD, Kotloff RM, Pochettino A, Arcasoy SM,
Rosengard BR, et al. (2003) Clinical risk factors for primary
graft failure following lung transplantation. Chest 124: 1232-
1241.
4. Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME,
et al. (2006) Risk factors for primary graft dysfunction after
lung transplantation. J Thorac Cardiovasc Surg 131: 73-80.
5. Kreisel D, Krupnick AS, Puri V, Guthrie TJ, Trulock EP, et
al. (2011) Short- and long-term outcomes of 1000 adult lung
transplant recipients at a single center. J Thorac Cardiovasc
Surg 141: 215-222.
6. Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, et
al. (2013) Clinical risk factors for primary graft dysfunction
after lung transplantation. Am J Respir Crit Care Med 187:
527-534.
7. Hayes D, Whitson BA, Ghadiali SN, Tobias JD, Mansour
HM, et al. (2015) Influence of HLA Mismatching on Survival
in Lung Transplantation. Lung 193: 789-797.
8. Ius F, Sommer W, Tudorache I, Gottlieb J, Haverich A, et al.
(2014) Early donor-specific antibodies in lung transplantation:
risk factors and impact on survival. J Heart Lung Transplant
33: 1255-1263.
9. Bharat A, Kuo E, Steward N, Aloush A, HachemR, et al.
(2008) Immunological link between primary graft dysfunction
and chronic lung allograft rejection. Ann Thorac Surg 86:
189-195;
10. Christie JD, Van Raemdonck D, de Perrot M, Barr M,
Keshavjee S, et al. (2005) Report of the ISHLT Working
Group on Primary Lung Graft Dysfunction part I: introduction
and methods. J Heart Lung Transplant 24: 1451-1453.
11. Christie JD, Carby M, Bag R, Corris P, Hertz M, et al. (2005)
Report of the ISHLT Working Group on Primary Lung Graft
Dysfunction part II: definition. A consensus statement of the
International Society for Heart and Lung Transplantation. J
Heart Lung Transplant 24: 1454-1459.
12. Diamond JM, Arcasoy S, Kennedy CC, Eberlein M, Singer
JP, et al. (2017) Report of the International Society for
Heart and Lung Transplantation Working Group on Primary
Lung Graft Dysfunction, part II: Epidemiology, risk factors,
and outcomes-A 2016 Consensus Group statement of the
International Society for Heart and Lung Transplantation. J
Heart Lung Transplant 36: 1104-1113.
13. Samano MN, Fernandes LM, Baranauskas JC, Correia AT,
Afonso Jr JE, et al. (2012) Risk factors and survival impact
of primary graft dysfunction after lung transplantation in a
single institution. Transplant Proc 44: 2462-2468.
14. Bonser RS, Taylor R, Collett D, Thomas HL, Dark JH, et
al. (2012) Effect of donor smoking on survival after lung
transplantation: a cohort study of a prospective registry.
Lancet 380: 747-755.
15. Pelaez A, Mitchell PO, Shah NS, Force SD, Elon L, et
al. (2015) The role of donor chronic alcohol abuse in the
development of primary graft dysfunction in lung transplant
recipients. Am J Med Sci 349: 117-123.
16. Lowery EM, Kuhlmann EA, Mahoney EL, Dilling DF,
Kliethermes SA, et al. (2014) Heavy alcohol use in lung
donors increases the risk for primary graft dysfunction.
Alcohol Clin Exp Res 38: 2853-2861.
17. Jacob S, Courtwright A, El-Chemaly S, Racila E, Divo M,
et al. (2016) Donor-acquired fat embolism syndrome after
lung transplantation. Eur J Cardiothorac Surg 49: 1344-1347.
18. Liu Y, Su L, Jiang SJ (2014) Recipient-related clinical risk
factors for primary graft dysfunction after lung transplantation:
a systematic review and meta-analysis. PLoS One 9: e92773.
19. Porteous MK, Lee JC, Lederer DJ, Palmer SM, Cantu E, et
al. (2017) Clinical Risk Factors and Prognostic Model for
Primary Graft Dysfunction after Lung Transplantation in
Patients with Pulmonary Hypertension. Ann Am Thorac Soc
14: 1514-1522.
20. Shah RJ, Diamond JM, Cantu E, Flesch J, Lee JC, et al.
(2015) Objective Estimates Improve Risk Stratification for
Primary Graft Dysfunction after Lung Transplantation. Am
J Transplant 15: 2188-2196.
21. Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA,
Zacharoulis D (2018) Extracorporeal membrane oxygenation
versus cardiopulmonary bypass during lung transplantation:
a meta-analysis. Gen Thorac Cardiovasc Surg 66: 38-47.
22. Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer
DJ, et al. (2009) Plasma levels of receptor for advanced
glycation end products, blood transfusion, and risk of primary
graft dysfunction. Am J Respir Crit Care Med 180: 1010-
1015.
23. Borders CF, Suzuki Y, Lasky J, Schaufler C, Mallem D, et
al. (2017) Massive donor transfusion potentially increases
recipient mortality after lung transplantation. J Thorac
Cardiovasc Surg 153: 1197-11203.e2.
24. Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino
A, et al. (2009) Risk factors for early primary graft dysfunction
after lung transplantation: a registry study. Clin Transplant
23: 819-830.
25. Alvarez A, Moreno P, Illana J, Espinosa D, Baamonde C, et
al. (2013) Influence of donor-recipient gender mismatch on
graft function and survival following lung transplantation.
Interact Cardiovasc Thorac Surg 16: 426-435.
26. Baldwin MR, Peterson ER, Easthausen I, Quintanilla I,
Colago E, et al. (2013) Donor age and early graft failure
after lung transplantation: a cohort study. Am J Transplant
13: 2685-2695.
27. Roubinian N, TACO, TRALI (2018) biology, risk factors, and
prevention strategies. Hematology Am Soc Hematol Educ
Program 2018: 585-594.
28. Ayala García MA, González Yebra B, López Flores AL, Guaní
Guerra E (2012) The major histocompatibility complex in
transplantation. J Transplant 2012: 842141.
29. Peltz M, Edwards LB, Jessen ME, Torres F, Meyer DM (2011)
HLA mismatches influence lung transplant recipient survival,
bronchiolitis obliterans and rejection: implications for donor
lung allocation. J Heart Lung Transplant 30: 426-434.
30. Chin N, Paraskeva M, Paul E, et al.: Comparative analysis
of how immune sensitization is defined prior to lung
transplantation. Hum Immunol 2015;76:711-6.
31. Smits JM, Mertens BJ, Van Houwelingen HC, Haverich A,
Persijn GG, et al. (2003) Predictors of lung transplant survival
in eurotransplant. Am J Transplant 3: 1400-1406.
32. Quantz MA, Bennett LE, Meyer DM, Novick RJ (2000)
Does human leukocyte antigen matching influence the
outcome of lung transplantation? An analysis of 3,549 lung
transplantations. J Heart Lung Transplant 19: 473-479.
33. Opelz G, Süsal C, Ruhenstroth A, Döhler B (2010) Impact of
HLA compatibility on lung transplant survival and evidence
for an HLA restriction phenomenon: a collaborative transplant
study report. Transplantation 90: 912-917.
34. Tsuang WM, Snyder LD, Budev MM (2020) Perspectives on
donor lung allocation from both sides of the Atlantic: The
United States. Clin Transplant 34: e13873.

