Recent Advances in the Translational Application of Immunotherapy with Pulsed Dendritic Cell-Derived Exosomes (DEX)
© 2024 Ramon Gutierrez Sandoval, Francisco Gutierrez Castro TM, Ider Rivadeneira, Francisco Krakowiak TM, Jordan Iturra, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This article explores recent advances in immunotherapy using exosomes derived from dendritic cells (DEXs), highlighting their potential as an innovative option in cancer treatment. DEXs have demonstrated the ability to activate robust and sustainable immune responses, overcoming the limitations of conventional therapies. Their low toxicity profile and capacity to induce long-term immunological memory position them as a viable alternative, especially for patients who do not respond to traditional treatments.
The article analyzes the mechanisms of action of DEXs and details their optimized production using advanced pulsing techniques. Clinical trials in melanoma, lung cancer, and other resistant tumors underscore their efficacy and the potential for combining them with conventional treatments, thereby improving tolerance and increasing effectiveness by minimizing adverse reactions.
Additionally, the article reviews the epicutaneous administration of DEXs, a strategy that enhances immune response while improving patient experience. The adaptability of DEXs to different types and stages of cancer makes them a fundamental tool in personalized oncology. The question is no longer whether this therapy is effective, but rather when and which low-cost implementation option will be chosen for clinical use, consolidating DEXs as an innovative and validated therapeutic line integrated into protocols that promote more precise and safer treatments with greater effectiveness.
Introduction
Cancer treatment has been a constant challenge in oncology due to the biological complexity of tumors and their ability to evade immune responses. In recent decades, immunotherapy has emerged as an effective alternative to conventional therapies, and within this category, dendritic cell-derived exosomes (DEXs) have shown particular promise [1].
Dendritic cells are central to the immune response due to their ability to present antigens to T cells, activating both the innate and adaptive immune responses. However, in the context of cancer, dendritic cells are often overwhelmed by the immunosuppression of the tumor microenvironment. Dendritic cell-derived exosomes offer a solution to this challenge, as they can be produced and optimized in the laboratory, allowing their loading with tumor- specific antigens through advanced pulsing techniques [2].
The use of exosomes as an immunotherapy platform has grown rapidly in recent years due to their ability to generate robust and sustained immune responses. Unlike conventional dendritic cell vaccines, exosomes are less susceptible to tumor immune evasion mechanisms, increasing their efficacy [3]. Furthermore, their ability to induce long-term immunological memory is a crucial advantage, preventing relapses and allowing the patient’s immune system to continue fighting any resurgence of cancer cells [4].
Exosome (DEX)-based immunotherapy is also remarkable for its adaptability to different treatment regimens and dosages. Thanks to its robust safety profile and highly favorable therapeutic index, DEXs allow flexibility in dosing, adjusting to the specific needs of each patient. This implies that doses can be administered periodically and in a personalized manner, without compromising the safety or efficacy of the treatment [5].
Additionally, DEXs have been shown to be effective through multiple routes of administration, such as intravenous, subcutaneous, intratumoral, intranodal, intranasal, and even epicutaneous routes (Figure A). This ability to be administered by various routes allows for greater precision in treatment, adapting to the particular characteristics of the tumor and optimizing the patient’s immune response [6,7]. This versatility not only improves therapeutic efficacy but also contributes to greater comfort for the patient, promoting adherence to treatment and increasing the chances of clinical success.
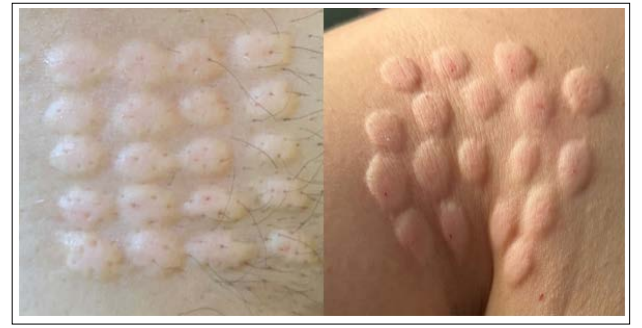
Figure A: The Epicutaneous or Intradermal Administration of Exosomes Derived from Dendritic Cells
Has been shown to be an effective and well-supported route for cancer immunotherapy. The study by Hao et al. (43) demonstrates that intradermal administration significantly outperforms subcutaneous delivery by promoting greater migration of dendritic cells to T-cell areas in the lymph nodes. This enhances the proliferation of CD8+ T-cells and improves the cytotoxic response, both key factors for an effective antitumor response.
Clinical trials have shown that DEXs may be particularly effective in treating melanoma, lung cancer, and advanced colon cancer, where conventional therapies have failed [8]. This article will explore recent advances in the mechanisms of action of DEXs, the optimization of their production using pulsed techniques, and how these developments are changing the landscape of cancer immunotherapy.
Benefits, Purposes, Expectations, and Mechanisms of Action of DEXs
Dendritic cell-derived exosomes (DEXs) act as efficient vehicles for tumor antigens, triggering the immune system to eliminate malignant cells in a targeted manner. These extracellular vesicles, ranging from 30 to 150 nanometers, contain key biomolecules such as proteins, lipids, and nucleic acids (RNA and DNA), essential for immunomodulation [9]. In DEXs, the presence of Major Histocompatibility Complex (MHC) class I and II proteins, costimulatory proteins such as CD86, and cytokines is critical to trigger an effective immune response.
DEXs also include tumor antigen-derived peptides, which act as “watchdogs” that alert the immune system to the presence of malignant cells. These peptides are processed by the patient’s T cells, allowing for a targeted immune response. The ability of exosomes to present antigens to T cells is key to amplifying the immune response in cases of difficult-to-treat tumors [10].
One of the most important mechanisms of DEXs is their ability to activate both CD4+ and CD8+ T cells, crucial elements of the adaptive immune system. CD8+ T cells, known as cytotoxic cells, are responsible for directly destroying tumor cells. DEXs, by carrying MHC class I molecules loaded with specific tumor antigens, interact with the receptors of CD8+ T cells, activating them and causing their proliferation. This activation results in the direct attack on malignant cells through the release of perforins and granzymes, which induce apoptosis [11].
In addition, DEXs are essential for the activation of CD4+ T cells or T helper cells. These cells coordinate the immune response by releasing cytokines that recruit and activate other types of immune cells, such as macrophages and CD8+ T cells. The interaction between MHC class II molecules present in DEXs and TCR receptors on CD4+ T cells amplifies the immune response, generating an effective and broad-spectrum immune action [12].
A key feature of dendritic cell-derived exosomes is their ability to induce immunological memory. Following activation of T cells by DEXs, some differentiate into memory cells, which remain in the body ready to respond rapidly in case the tumor recurs [13]. This prolonged immunological memory is crucial to prevent relapses, providing a significant advantage over conventional treatments, which do not offer long-term protection.
In addition to activating a specific immune response, DEXs can influence the tumor microenvironment, an immunosuppressive environment that often prevents the effective attack on cancer cells. DEXs release cytokines and other mediators that modulate this microenvironment, affecting immunosuppressive cells such as tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) [14]. By reversing the immunosuppressive state, DEXs promote a more favorable environment for cytotoxic T cells and other immune system effector cells.
Another key benefit of DEXs is their ability to interfere with angiogenesis, the process by which tumors develop new blood vessels to grow and spread. By interfering with this process, DEXs limit tumor growth and make it more vulnerable to immune destruction [15]. This dual impact, both in modulating the microenvironment and inhibiting angiogenesis, underlines the therapeutic potential of DEXs against cancer, while their ability to induce immunological memory makes DEXs not only effective in triggering an initial immune response but also in maintaining a favorable environment in the long term, which improves treatment efficacy and reduces the risk of relapse. This advantage, together with their ability to act on resistant tumors, offers a promising perspective compared to conventional therapies [16].
A highlight of the use of DEX in combination with conventional therapies such as chemotherapy is its ability to reduce up to 50% of the adverse reactions associated with these treatments. By improving the modulation of the immune system and reducing overall toxicity, DEXs increase patient tolerance, allowing them to complete the planned therapeutic cycles [17]. This benefit not only improves the patient’s quality of life but also increases the success rates of conventional therapies by reducing the number of treatment dropouts or interruptions.
>Optimization in Immunotherapy: Dendritic Cell Pulsing, Quality Control, and Functional Assessment in Advanced Therapies
The pulsing of dendritic cells (DCs) allows them to present specific antigens to the immune system, stimulating cytotoxic T cells that recognize and destroy tumor cells. The choice of antigen sources and the mechanisms for introducing them into DCs are critical to the quality of the immune response and, therefore, to the success of the therapy.
Autologous biopsy, obtained directly from the patient’s tumor, is one of the most specific sources of antigens. This approach ensures that DCs are loaded with neoantigens unique to the tumor, generating a highly personalized immune response. However, this method is not without limitations. During processing, the tissue is subjected to chemicals such as formalin, alcohol, and xylene, and is subjected to high temperatures during paraffin embedding, which can alter the antigenic structure [18]. To mitigate these effects, antigen retrieval techniques are used, although these are not always completely effective. Therefore, autologous biopsy should not be considered the only approach, especially when factors such as costs and material quality influence the success of the therapy [5].
The use of heterologous biopsies or tumor bank material is a viable alternative when an adequate autologous sample cannot be obtained. This method has the additional benefit of increasing the antigenic variability profile, which can more robustly stimulate the patient’s immune response by providing a broader range of tumor epitopes. Thus, the antigenic diversity it offers can be advantageous for inducing a stronger and more effective immune response. This type of approach, which uses tumor lysates from similar tumors, has been shown to be effective in contexts where the autologous profile is not critical or is logistically unfeasible [19].
Cultured tumor cell lines, on the other hand, offer a controlled and standardized source of antigens, particularly useful in clinical studies and large-scale production. These lines are subjected to lysis processes that release proteins and antigenic fragments, without being exposed to the effects of toxic chemicals or thermal variations [18]. This consistency allows greater control of antigen quality, which is essential for multicenter trials, constituting an efficient option for the generation of an anti-tumor immune response [19].
Another promising strategy is the use of synthetic peptides, which replicate specific antigenic fragments, allowing a targeted response against key mutations in tumors. This approach is highly controlled and avoids the difficulties associated with tissue processing, improving the specificity of the immune response [7]. Studies have shown that these peptides can be introduced into DCs by co- culture or advanced techniques such as electroporation, producing significant immune responses in patients with solid tumors [6].
Clinical Impact: Evaluation by iRECIST
Accurate evaluation of the effectiveness of this treatment must be based on solid clinical parameters that, in addition to measuring tumor response, consider the recovery of the patient’s quality of life, a primary objective in this type of intervention. One of the essential methods to monitor the patient’s immune response during DEX treatment is the measurement of interleukins in peripheral blood. These cytokines play a crucial role in regulating the immune response and can offer valuable information on how the immune system is reacting to immunotherapy. Specialized laboratories with the capacity to perform flow cytometry equipped with appropriate panels, are necessary to measure the immunophenotyping analysis (Figure B), analysis of cell viability (Figure C) variability of interleukins throughout the treatment. This analysis allows comparing levels before, during, and after treatment, providing key data on the activation of the immune system. Previous studies have shown that DEX can influence the release of interleukins such as IL-2 and IFN-γ, both indicators of an active and effective immune response against the tumor [12].
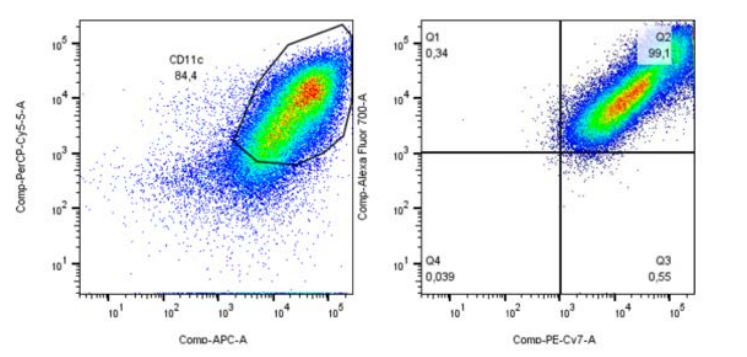
Figure B: Reference Image of Immunophenotypic Analysis using Flow Cytometry
Crucial for evaluating the quality and effectiveness of dendritic cell-based immunotherapy in cancer patients. In figures like the one analyzed, it is possible to consider approximately 100,000 events and then identify the significant fraction of Lin1 (-) cells, confirming the appropriate cell selection for treatment. The percentage of myeloid dendritic cells and mature dendritic cells can be identified using anti-CD123 and HLADR markers for the myeloid population, and CD11C and HLADR for the lymphoid population. Additionally, markers such as CD80 and CD83 ensure that the cells are functional and capable of activating T cells.
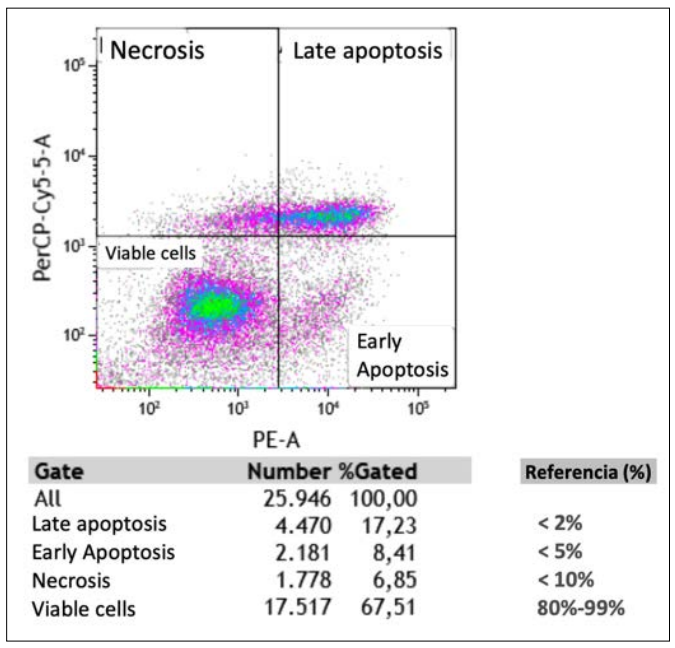
Figure C: Reference Image of Cell Viability Analysis Using Flow Cytometry
In the context of dendritic cell-based immunotherapy for cancer, cell viability analysis through flow cytometry provides a crucial evaluation of the sample’s quality and functional capacity. Analyses like the one illustrated reveal cell viability over approximately 26,000 events, allowing the establishment of a high percentage of active and functional dendritic cells.
In parallel, clinical monitoring can be complemented with advanced imaging studies, such as PET-CT with 18FDG, which allows the visualization and quantification of the metabolic activity of the tumor using its SUV indicator (Standardized Uptake Value). This is essential for determining cancer progression or regression during treatment, including the challenge of recognizing pseudo- progression phenomena. This phenomenon is characterized by apparent tumor growth due to infiltration of immune cells, which can be misinterpreted as a worsening of the disease. To avoid this confusion, criteria such as iRECIST are used, which allow differentiation between true progression and atypical responses, thus adjusting the assessment of treatment in real-time [20,21].
Exosome therapy requires a comprehensive approach that combines clinical measurements, immunological studies, and advanced imaging techniques. Monitoring interleukin levels (Figure D,E), conducting PET-CT scans, and applying specific evaluation criteria such as iRECIST are essential to optimize therapy, enhance immune response, and reduce the risk of relapse. These components are crucial in ensuring that DEX immunotherapy not only effectively controls tumors but also improves the patient’s quality of life.
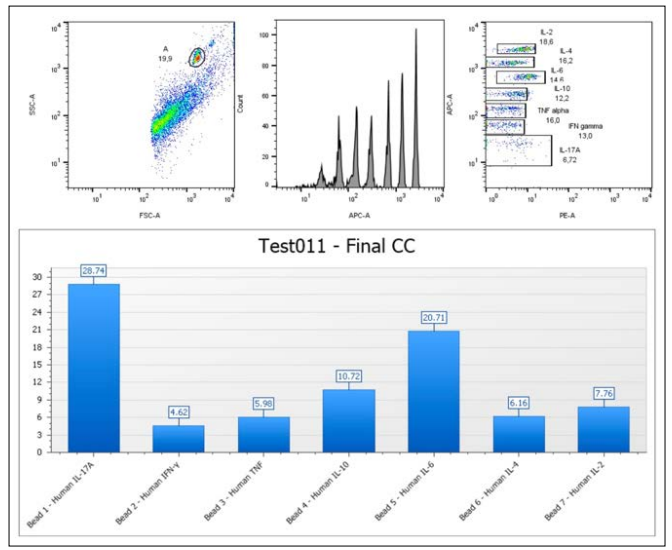
Figure D: Reference Image of Dot Plot Analysis of Blood Plasma
Dot plot analysis of blood plasma, based on the identification of CBA Th4-Th2-Th47 markers, provides a key assessment of the patient’s immune response during dendritic cell immunotherapy. Cytokines, measured in picograms (pg/ml), top standard (5.000 pg/ml), such as IFNγ, TNFα, IL-10, IL-6, IL-4, IL-2, and IL- 17A, reflect the balance between pro-inflammatory (Th4) and anti-inflammatory (Th2) responses, as well as Th47-associated inflammation. This balance is crucial for monitoring therapy effectiveness, as it indicates whether the immune system is activating the cytotoxic T cells necessary to destroy the tumor while controlling inflammation levels that could negatively impact the patient. This type of analysis allows for treatment adjustments and optimization, providing a more precise and effective therapy.
This profile reflects the balance between pro-inflammatory (Th4), anti-inflammatory (Th2), and Th47-associated inflammation, all crucial for tumor surveillance and control. Quantifying these cytokines through a calibration curve helps assess the effectiveness of immunotherapy. For instance, IFNγ and TNFα are markers of a strong Th4 response that activates cytotoxic T-cells, essential for destroying cancer cells. IL-10 and IL-4 regulate inflammation and may indicate immune suppression, while IL-17A, depending on the context, can either enhance anti-tumor immunity or promote tumor growth.
This analysis not only evaluates the immune system’s response to treatment but also informs therapy optimization. A balanced Th4/ Th2/Th47 profile is critical for maximizing the immune response against the tumor while avoiding autoimmunity or uncontrolled inflammation. Clinicians can adjust the therapeutic strategy based on these results, aiming for a more effective and precise immune response.
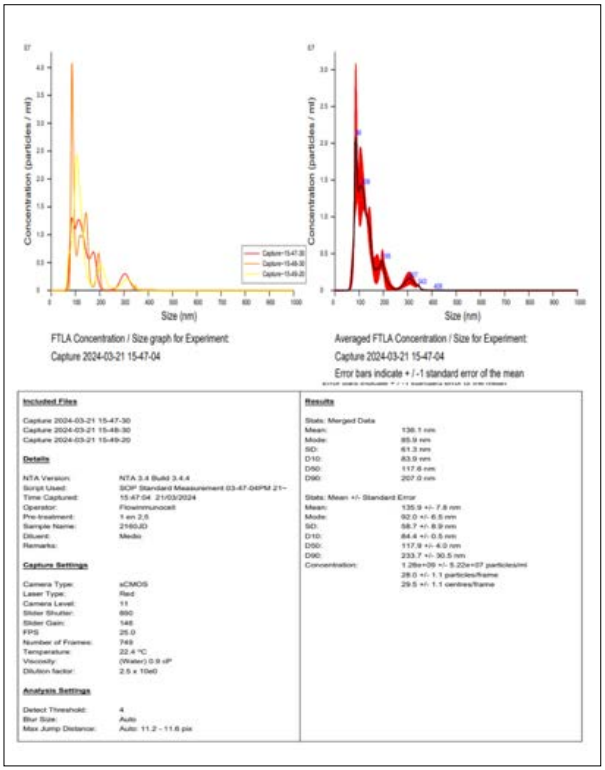
Figure E: Reference Image of Nanosight Analysis
This analysis provides critical information on the quality and suitability of the exosome sample used in dendritic cell-based immunotherapy for cancer. Exosomes, predominantly sized at 86 nm, are within the optimal range for transporting tumor antigens, which is essential for stimulating the immune system. By enhancing T cell activation, these exosomes play a key role in promoting an effective immune response against cancer cells. The sample concentration, measured in millions of nanoparticles per ml, demonstrates its potency, ensuring a sufficient quantity of exosomes to trigger a robust immune reaction. A high level of observable purity (1.1 factor) confirms that the sample consists primarily of exosomes, free of other contaminants or microvesicles that could interfere with the therapeutic effect.
The sample’s concentration of 1.280 billion nanoparticles per ml demonstrates its potency, ensuring that a sufficient number of exosomes are present to trigger a robust immune reaction. The high purity level (factor of 1.1) further confirms that the sample consists mainly of exosomes, free from other contaminants or microvesicles that could hinder the therapeutic effect.
In conclusion, the sample shows excellent purity, homogeneity, and concentration, making it highly suitable for use in dendritic cell immunotherapy. These characteristics ensure that the exosomes can efficiently communicate with the patient’s immune system, enhancing the overall effectiveness of the cancer treatment. The precise characterization through the Nanosight NS300 ensures that the sample is optimized for clinical application, providing a reliable basis for a targeted and potent immunotherapeutic response.
In this context, Nanosight technology plays a pivotal role in assessing the integrity and quality of exosomes used in dendritic cell-based immunotherapy. This system provides key data on exosome concentration, size, and distribution, ensuring that the sample is suitable for clinical application [22,23]. The analysis shows that the exosome sample contains a high concentration of nanoparticles (1.280 billion per ml) with a predominant size of 86 nm, which is optimal for carrying tumor antigens and stimulating the immune system.
The precision of Nanosight in measuring the purity and homogeneity of exosomes is critical for ensuring their therapeutic efficacy. By confirming the integrity of the exosome sample, this technology supports the effectiveness of immunotherapy by enhancing the immune system’s ability to target tumor cells. This comprehensive evaluation process helps optimize the clinical benefits of exosome therapy, ensuring safety and maximizing long-term outcomes while minimizing the risk of relapse. [22,23].
Relationship and Comparison with Conventional Treatments Conventional cancer treatments, such as chemotherapy and radiotherapy, have been the mainstays of oncological management for decades. Despite being effective, they present significant limitations in some cases, particularly in patients with advanced- stage cancers or those who have experienced relapses after initial treatment. The main disadvantage of these therapies is their lack of specificity, resulting in the destruction of not only tumor cells but also healthy cells [5,6]. This generates serious side effects, such as immunosuppression, cardiotoxicity, neurotoxicity, and an increased risk of severe infections due to suppression of the immune system [2].
Chemotherapy works by interfering with the cell cycle, attacking rapidly proliferating cells. However, healthy tissues, such as bone marrow and gastrointestinal epithelium, are also vulnerable to this attack, leading to immunosuppression and other debilitating effects [11]. Furthermore, the cumulative toxicity of chemotherapy may eventually prevent continued treatment, despite the presence of residual cancer [16]. Radiotherapy, although more localized, also damages surrounding tissues, especially when tumors are close to vital organs, such as the brain or heart, which can aggravate side effects and reduce the patient’s quality of life [24].
On the other hand, dendritic cell-derived exosomes (DEXs) have emerged as a much more precise therapeutic alternative, as they induce a specific immune response against tumor cells, significantly reducing damage to healthy tissues [25]. Unlike conventional treatments, DEXs present specific tumor antigens to T cells of the immune system, triggering a highly targeted immune response. This process not only eliminates cancer cells but also establishes a long-term immunological memory, crucial for preventing tumor recurrence [26]. Furthermore, DEXs do not induce tumor resistance, making them a viable option for patients with recurrent or treatment-resistant cancers [23].
DEXs not only reduce tumor burden without the devastating side effects of chemotherapy but also significantly improve the patient’s quality of life [11]. By inducing a prolonged immune response, DEXs allow for continuous monitoring of the immune system, which is essential to reduce the rate of cancer recurrence [24]. DEXs have shown particular effectiveness in patients who do not tolerate conventional treatments or in cases of stage 4 cancer, in which therapeutic options are limited [6].
Unlike traditional treatments, which are often limited by cumulative toxicity, the side effects of DEXs are markedly milder. Patients treated with DEXs tend to experience only local injection site reactions or flu-like symptoms, in contrast to the profound immunosuppression, hair loss, and incapacitating nausea that often accompany chemotherapy [27]. This low toxicity also allows DEXs to be used in combination with other treatments, such as immune checkpoint inhibitors, increasing the efficacy of treatments and opening the door to new therapeutic strategies [26].
Regarding the role of surgery, DEXs are not only changing the management of advanced and recurrent cases but also allow reconsideration of surgery as a less invasive option when combined with preoperative immunotherapy. By reducing tumor burden without the toxicity of chemotherapy, patients arrive at surgery in better condition, increasing the chances of surgical success [25]. Furthermore, DEXs contribute to preventing postoperative metastatic spread by strengthening the immune response to destroy any residual tumor cells that may have been released during surgery [7]. Taken together, DEXs are revolutionizing not only cancer treatment but also the way traditional therapies are delivered.
Recent Advances in DEX Research
Research on dendritic cell-derived exosomes (DEXs) has advanced significantly in recent years, particularly in combination with immune checkpoint inhibitors, such as anti-PD-1 and anti- CTLA-4 antibodies. These combinations have shown remarkable improvement in response rates for metastatic cancers [25]. DEXs not only function independently but also enhance the efficacy of other immunotherapies. By unblocking the immune response suppressed by tumor cells, DEXs offer additional therapeutic benefits [28].
One of the most revolutionary aspects of DEXs is their ability to modulate the immune system specifically and durably. Clinical trials have demonstrated their effectiveness, with the combination of DEXs and checkpoint inhibitors doubling overall survival rates in patients with metastatic melanoma, achieving a median survival of 24 months compared to 12 months with conventional chemotherapy alone [26].
The synergy between DEXs and immune checkpoint inhibitors is due to their activation of cytotoxic T cells, essential for tumor destruction. While inhibitors unshackle the immune system’s ability to attack tumors, DEXs present tumor-specific antigens, amplifying the immune response and maintaining sustained action. This is especially important in “immunologically cold” tumors, where DEXs activate a broader range of T cells [7].
In addition to improving survival rates, DEXs in combination with checkpoint inhibitors significantly reduce tumor burden, allowing patients to live longer with a higher quality of life. This approach has shown to be particularly effective in cancers resistant to traditional treatments, reinforcing DEXs as a transformative tool in next- generation immunotherapy [19].
One major challenge in cancer treatment is minimal residual disease (MRD)—residual tumor cells that persist after initial treatments and can cause relapses months or years later. DEXs, by inducing a specific immune response against tumor antigens, are capable of eliminating these residual cells [6]. Clinical trials have shown that patients treated with DEXs had 40% less MRD compared to those who received conventional therapies, significantly reducing the risk of relapse [8].
This development is especially critical for aggressive and metastatic cancers, where eliminating MRD reduces the chance of recurrence and improves the likelihood of long-term remission [24]. Furthermore, DEXs establish immunological memory, preparing the immune system to respond swiftly if any tumor cells reappear in the future, offering lasting protection [7].
Another vital feature of DEXs is their immunoplasticity, which refers to their ability to adapt to various immune environments and cancer types [2]. DEXs can be tailored to address the specific characteristics of each tumor, making them a versatile therapeutic tool. This adaptability is especially useful in treating solid tumors such as those found in breast, lung, and colon cancers, as well as metastatic cancers [4]. DEXs can target multiple tumor sites simultaneously, making them a valuable option for patients with metastases [19].
DEXs also show remarkable flexibility in different cancer stages. In early stages, they can serve as adjunctive treatments, enhancing the efficacy of surgery or chemotherapy by eliminating residual tumor cells [6]. In advanced stages, DEXs reduce tumor burden and improve patient quality of life, as they do not induce the severe side effects commonly associated with conventional treatments [28].
Technological Innovations in DEX Production
One of the primary challenges in the production of dendritic cell-derived exosomes (DEXs) has been scaling the process to produce sufficient quantities for widespread clinical use. Recent innovations, such as the implementation of bioreactors and the integration of artificial intelligence (AI) into production protocols, have significantly improved the scalability, quality, and efficiency of DEX production. These advancements have made it possible to generate DEXs on a much larger scale, enabling treatment for a broader range of patients [29,30].
Genetic engineering has also seen breakthroughs that enhance the therapeutic efficacy of DEXs. For example, researchers are now able to load exosomes with greater quantities of tumor antigens or molecules that can penetrate solid tumors more effectively, thereby enhancing the exosomes’ ability to activate the immune system. This strategy not only improves the overall therapeutic outcomes but also helps reduce production costs, making the therapy more accessible [16,19].
In addition, the integration of genetic editing tools such as CRISPR-Cas9 has shown promise in further enhancing DEX functionality. By leveraging this technology, researchers can improve the specificity and adaptability of DEXs to different types of cancer. Furthermore, the use of biomarkers has allowed for more precise patient selection, enabling clinicians to identify those who are most likely to benefit from DEX-based immunotherapy, improving both treatment efficacy and patient outcomes [28].
Challenges, Ethical Implications, and Access
As technological advances enable increased production and efficacy of dendritic cell-derived exosomes (DEXs), several challenges arise in ensuring that these therapies are both safe and accessible to a broader patient population. One of the primary concerns lies in the regulation and oversight of clinical trials for DEX-based therapies. Proper clinical guidelines are essential to define how DEXs should be integrated with other therapies, such as immune checkpoint inhibitors, radiotherapy, or chemotherapy [20]. Without this guidance, there is a risk that treatments could be used inconsistently, leading to variable outcomes and potential risks for patients.
The ethical implications of DEXs also warrant careful consideration. Although significant strides in biotechnology have lowered production costs, the complex nature of exosome-based treatments still makes them relatively expensive compared to conventional therapies. This cost barrier raises issues of equitable access, especially in regions with limited healthcare resources. To ensure that DEX-based therapies are available to all patients regardless of their geographic location or socioeconomic status— governments, researchers, and pharmaceutical companies must collaborate to develop strategies for widespread access [19].
Additionally, the development of global health policies that promote equal access to advanced therapies like DEXs is critical. As the production and clinical use of DEXs expand, policymakers must address questions surrounding intellectual property rights, the affordability of treatments, and the allocation of healthcare resources [28].
Despite these challenges, continued advances in biotechnology are expected to drive down production costs, making DEXs a more viable treatment option for a wider variety of cancers. Their adaptability to different tumor profiles and their capacity for customization to the specific needs of patients further underscore their potential as a standard of care in the near future [19,28].
Conclusions
Exosome-based immunotherapy derived from pulsed dendritic cells (DEX) has radically transformed the approach to cancer treatment. Initially considered experimental, this therapy has advanced significantly, validated through numerous well-known clinical trials [31]. However, much of the research and development has now shifted to international corporations, where dissemination of information may be more restricted [32]. Research reports confirm that DEX has surpassed the preclinical phase and is now employed in advanced clinical trials for various types of cancer [33]. These trials have demonstrated not only the efficacy of DEX but also its ability to synergize with conventional therapies such as chemotherapy and radiotherapy as well as immune checkpoint inhibitors like PD-1 and CTLA-4, creating therapeutic synergy that enhances patient outcomes [24].
One of the most notable features of DEX is its capacity to generate long-term immune memory, contributing to reduced relapse rates [34]. Additionally, its low toxicity profile makes it an ideal option for patients who cannot tolerate the adverse effects of conventional treatments [12]. As a result, DEX is now recognized not just as a potential treatment, but as a critical option in the care of any cancer patient, regardless of the disease stage [35].
The primary challenge now is not whether patients should receive DEX, but rather determining which therapeutic combinations are most effective for each type of cancer and clinical context [36]. Furthermore, studies have shown that DEX is particularly effective in targeting minimal residual disease (MRD), significantly reducing the risk of relapse, which is critical for patients with metastatic cancer [37].
Thus, DEX immunotherapy represents an essential tool for the future of personalized and precision cancer treatment [38]. The continuous development and optimization of therapeutic combinations with other immunological and conventional treatments open new frontiers in cancer management, ensuring that DEX solidifies its position as a key pillar of modern cancer therapy [39]. The future of DEX immunotherapy will also rely on proper guidance and education from the medical team to patients, preventing self-managed decisions, which are becoming increasingly common. The ease of administration through the epicutaneous route further enhances the possibility of home- based care, ensuring a high level of safety and effectiveness [40]. Finally, the availability of centers with access to biotechnology, scalable production lines, and lower costs, while maintaining the personalization and precision of therapeutic protocols, is another key factor in the ongoing success of DEX immunotherapy [41-43].
References
- Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and Nat Rev Immunol 2: 569-579.
- Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14: 195-208.
- Tkach M, Théry C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164: 1226-1232.
- André F, Schartz NE, Movassagh M, Mojgan Movassagh, Caroline Flament, et al. (2002) Malignant effusions and immunogenic tumor-derived Lancet 360: 295-305.
- Horneber M (2014) Chemotherapy for cancer: current and future perspectives. Cancer Chemother Pharmacol 73: 463-
- Phelan C (2017) The toxicities of cancer treatment: a J Oncol Pract 13: 6.
- D’Angelo SP (2018) Long-term survival in patients with advanced melanoma treated with a combination of immunotherapy and targeted therapy. J Clin Oncol 36: 7002-
- Chen L, Dung T Le, Jennifer N Uram, Hao Wang, Bjarne R Bartlett, et (2018) PD-1 blockade in tumors with mismatch- repair deficiency. N Engl J Med 372: 2509-2520.
- McGowan E (2018) Clinical trial design in cancer immunotherapy: lessons learned from checkpoint inhibitors. Cancer Immunol Immunother 67: 1-10.
- Markman M (2019) Intellectual property rights and the development of cancer Clin Cancer Res 25: 3580- 3586.
- Balch CM (2013) Efficacy of immunotherapy in the treatment of melanoma. J Clin Oncol 31: 2-7.
- Baskar R (2012) Cancer and radiation therapy: current advances and future directions. Front Oncol 2: 72.
- Ascierto PA, Raffaele Addeo, Giacomo Cartenì, Bruno Danieleet, Michele De Laurentis, et al. (2014) The role of immunotherapy in J Transl Med 12: 145.
- Flaherty KT, Jeffery R Infante, Richard F Kefford, Adil Daud, Rene Gonzalez, et (2012) Effect of combined BRAF and MEK inhibition on the response of melanoma to treatment. N Engl J Med 367: 1694-1703.
- Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular Annu Rev Cell Dev Biol 30: 255-289.
- Zitvogel L, Regnault A, Lozier A, J Wolfers, C Flament, et (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4: 594-600.
- Chaput N, Schartz NE, André F, Caroline Flament, Nathalie Aubert, et al. (2004) Exosomes as potent cell-free peptide-based vaccines. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol 172: 2126-2136.
- Escudier B, Dorval T, Chaput N, Fabrice André, Marie-Pierre Caby, et al. (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell-derived exosomes: a phase I trial. J Transl Med 3: 10.
- Whiteside TL (2016) Exosomes and tumor-mediated immune J Clin Invest 126: 1216-1223.
- Ludwig N, Rubenstein LJ, Whiteside TL (2018) Exosomes in cancer: potential role in diagnosis, prognosis, and Biochim Biophys Acta Rev Cancer 1869: 145-152.
- Valadi H, Ekström K, Bossios A, Margareta Sjöstrand, James J Lee, et al. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between Nat Cell Biol 9: 654-659.
- Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200: 373-
- Hoshino A, Costa-Silva B, Shen TL, Goncalo Rodrigues, Ayako Hashimoto, et al. (2015) Tumor exosome integrins determine organotropic Nature 527: 329-335.
- Colombo M, Moita C, van Niel G, Joanna Kowal, James Vigneron, et al. (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126: 5553-5565.
- Bebelman MP, Smit MJ, Pegtel DM, Baglio SR (2018) Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther 188: 1-11.
- Guo W, Gao Y, Li N (2021) Dendritic cell-derived exosomes in cancer therapy: from bench to clinical Front Immunol 12: 716233.
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, et (2017) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17: 97-111.
- Chen L, Flies DB (2013) Molecular mechanisms of T cell co- stimulation and co-inhibition. Nat Rev Immunol 13: 227-242.
- Clayton A, Tabi Z (2005) Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis 34: 206-213.
- Pardoll DM (2012) The blockade of immune checkpoints in cancer Nat Rev Cancer 12: 252-264.
- Nishino M (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14: 655-668.
- Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, et al. (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first-line chemotherapy in NSCLC. Oncoimmunology
- Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9: 581-593.
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, et (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3: 9.
- Viaud S, Ploix S, Lapierre V, Théry C, Commere PH, et al. (2011) Updated review of Dendritic Cell-derived Exosomes (DEX) in cancer Cancer Res 71: 5317-5327.
- Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle J Extracell Vesicles 3: 24641.
- Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO (2016) Extracellular vesicles and intercellular communication within the nervous J Clin Invest 126: 1198-1207.
- Hsu DH, Paz P, Villaflor G, Rivas A, Mehta-Damani A, et (2003) Exosomes as a tumor vaccine: Enhancing the immune response to cancer. BMC Cancer 3: 19.
- Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33: 419-440.
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, et (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7: 297-303.
- Hao S, Ye Z, Yang J, Bai O, Xiang J (2006) Intradermal vaccination of dendritic cell-derived exosomes is superior to a subcutaneous one in the induction of antitumor Cancer Biother Radiopharm 21: 146-154.
- Pitt JM, Charrier M, Viaud S, André F, Besse B, et (2014) Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J Immunol 193: 1006-1011.
- Lener T, Mario Gimona, Ludwig Aigner, Verena Börger, Edit Buzas, et al. (2015) Applying extracellular vesicles-based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles 4:

