Immunohistochemical Pattern and Diagnostic Applications of Alpha-Methyl Coa Racemase and Anti-Carcinoembryonic Antigen on Prostate Cancer
© 2023 Okorie Nnaemeka, Ibe Uzochukwu Chimdindu, Simon Imakwu Okekpa, Ikenna Chinedu Ugochukwu, Akpukwa Udemezue Joseph, et al., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Prostate cancer is one of the most common types of malignant tumors, and its incidence rate has increased worldwide. It is the most prevalent cancer among men, most affecting older men, particularly those above 60 years of age. Notably, early detection, diagnosis, and treatment can result in improved patient outcomes, such as an improved 5-year survival rate. This study aims to evaluate the immunohistochemical pattern and diagnostic applications of alpha-methyl Co-A, racemase, and anti-carcinoembryonic antigen on prostate tumors. A total of one hundred (100) tissue blocks used in this study were collected from the archives of the histopathology laboratory at AEFUTHA and evaluated using Haematoxylin and Eosin Periodic Acid Schiff staining techniques and immunohistochemical stains. The hospital data show that a total of 132 prostate tumor samples were submitted for histological analysis from 2019 to 2021. Histologic sections of the prostatic tissues showed well-formed prostatic glands lined by two layers of low cuboidal cells to columnar epithelium, the presence of glandular secretions or corpora amylacae, and inflammatory infiltrates that were mostly made up of mononuclear cells. Also seen were poor attempts at glandular formation; glands were lined by a single layer of epithelial cells, of which the linning epithelial cells were pleomorphic with hyperchromatic nuclei and prominent nucleoli. Furthermore, evidence of tumour infiltration and spread, which included perineural invasion and stromal desmoplastic. The immunohistochemical staining reactions of AMACR and CEA in the tissue sections show various intensities of expression. The intensity of expression was scored in four levels: negative (0/3), weak expression (1/3), moderate expression (2/3), and strong expression (3/3). This present study showed that these markers are useful in the diagnosis of prostate cancer.
Introduction
Prostate cancer (PCa) is one of the most common types of malignant tumors, and its incidence rate has increased worldwide [1]. After non-melanoma skin tumors, PCa is the most prevalent cancer among men [2]. Predominantly among those above 60 years of age. The early clinical symptoms of PCa are difficult to detect [3]. and the sensitivity of the serum marker prostate- specific antigen (PSA) is low, which can result in false-negative test results [4]. Also, it has poor specificity for cancer, is highly expressed in noncancerous prostatic tissues as well as in cancerous tissues, and often leads to overdiagnosis and overtreatment [5]. These challenges necessitate the use of a new approach to identify possible high-risk PCa to better assist clinical decisions. Alpha- Methylacyl-CoA racemase (AMACR) is a novel marker that is positive in malignant epithelial prostatic carcinoma cells [6].
AMACR is an enzyme currently used in prostate cancer diagnosis; it is a peroxisomal and mitochondrial enzyme that was preferentially overexpressed in approximately 80% of prostate cancer detected in prostate biopsies [5]. On the same note, there are tumor-associated antigens (TAAs) expressed on the surface of cancer cells. Differences in the expression levels of TAAs in cancer cells compared to normal cells have led to these antigens being investigated as diagnostic and prognostic biomarkers. One of such TAAs is a carcinoembryonic antigen (CEA), also known as CEACAM5, and originally described as an oncofetal protein in colorectal cancer. It is present at low levels in adult tissues of epithelial origin, such as the colon, stomach, tongue, cervix, and prostate. Its overexpression and the change in its pattern of expression are indicative of tumor transformation. While in non- tumor cells CEA is restricted to the apical surface, in cancerous cells it appears dislocated all over the cellular membrane, contributing to its secretion [7].
In these tough situations, immunohistochemical stains of some basal cell markers may help with the diagnosis. Several markers, including AMACR and CEA, have been introduced and tested for utility [8]. In prostate cancer, immunohistochemistry has an important role in borderline cases due to the presence (or absence) of basal cells, detected by specific antibodies against them combined with racemase expression in luminal epithelial cells [9]. Typically, the diagnosis of high-grade prostate tumors is based on the morphological features (single cell layer and nuclear atypia) identified by H&E staining [3]. However, certain ambiguous cases, such as glandular basal cell hyperplasia, gland atrophy, clear cell degeneration, and squamous metaplasia, require confirmation via immunolabeling [10]. Thus, this study aims to investigate the diagnostic values of AMACR and anti-CEA antibodies by measuring their expression levels in PCa tissues using immunohistochemistry (IHC) analysis.
Materials and Methods
This research was done at the Alex Ekwueme Federal University Teaching Hospital, Abakaliki (AEFUTHA), Ebonyi State, Nigeria. This study is a cross-sectional study designed to examine formalin-paraffin-processed archived prostate tumor tissues for the expression of alpha-methyl CoA racemase and anti- carcinoembryonic antigen. Corresponding patients’ information was collected from the laboratory registry. The tissue blocks were formalin-fixed and paraffin-embedded prostate tumor tissues collected during routine diagnostic procedures in the hospital. These tissue blocks are banked in histopathology laboratories and made available for re-evaluation and research purposes. The tissue blocks then followed microtomy to obtain thin sections for staining.
The sections floated in 20% alcohol into the water bath using clean, grease-free glass slides, and then properly dried in hot air for proper adhesion. Tissue sections obtained from each block, were attached to three microscope slides for routine staining (H&E), and Periodic Acid Schiff respectively, in line with the procedures described by Okorie [11]. The remaining slides demonstrate the immune-histochemical expression patterns using immune- histochemical methods [12]. The sample size was calculated using the formula for sample size estimation for cross-sectional forms of observational studies with a defined population [13]. A total of one hundred (100) tissue blocks used in this study were obtained from archives of the histopathology laboratory at AEFUTHA.
Results
The data collected from the hospital show that a total of 132 prostate tumor samples were submitted for histological analysis from 2019 to 2021. One hundred (100) of these were sampled in this study. The associated demographic data are presented in tables. Histological analysis of the prostate tumor sections with PAS and H&E stains showed some features, including well-differentiated columnar glandular epithelial cells, concretions, inflammatory cells, poorly differentiated glands, hyperchromatic pleomorphic nuclei, perineural invasion, infiltration of stromal muscles, and complete loss of stroma, among others. These are presented below in plates. The immunohistochemical staining reactions of AMACR and CEA in the tissue sections show various intensities of expression. The intensity of expression was graded in four levels: negative (0/3), weak expression (1/3), moderate expression (2/3), and strong expression (3/3). The immunohistochemical expressions are presented for visualization in the plates below.
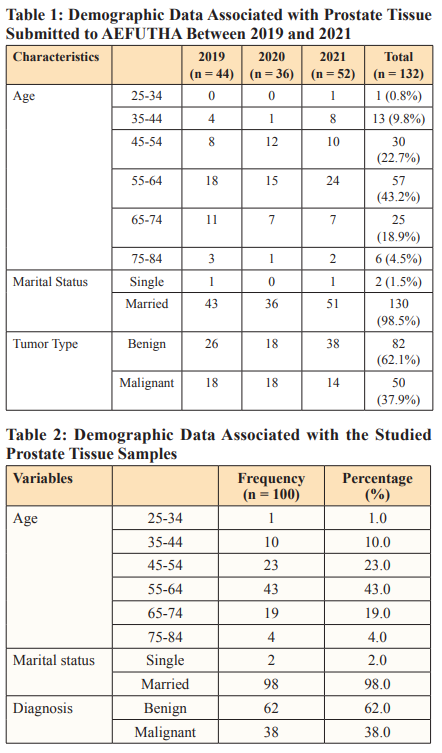
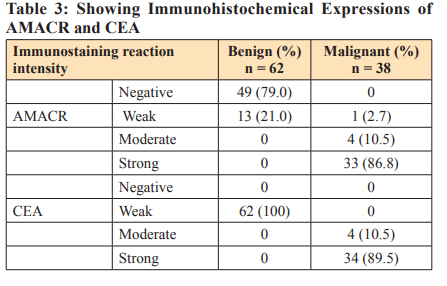
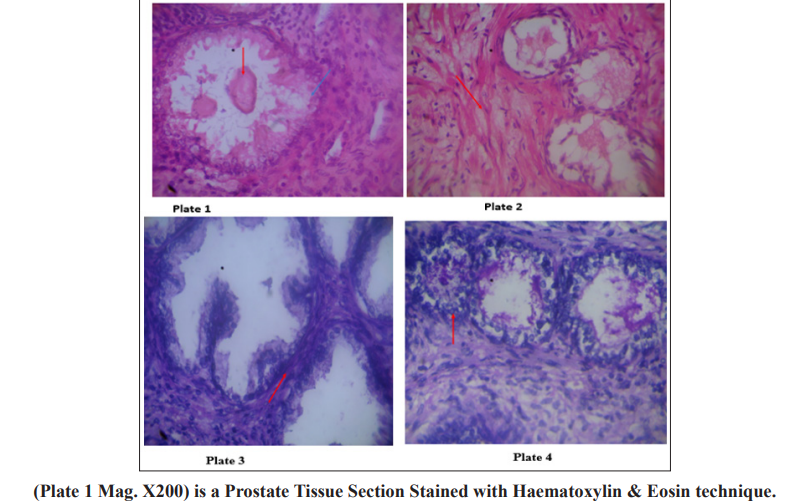
Section shows well differentiated columnal glandular epithelial cells (blue arrow) cells, cuboidal basal and two concretions or corpora amylacae (red arrow) which are typical of benign prostate hyperplasia seen in most aged men with stromal fibromuscular tissues. Plate 2 (Mag. X200) is a prostate section stained with Haematoxylin & Eosin. Section shows crowded varied glands arrangement, stromal fibromuscular tissues cellular, the fibromuscular stroma (red arrow) shows how reactive the stroma is to the atypical glands. The glands are lined by a single layer of cells. The lining cells are pleomorphic with hyperchromatic nuclei. This is indicative of prostate adenocarcinoma. Plate 3 (Mag. X200) is a prostate tissue stained with periodic acid Schiff reagents (PAS). Section shows uniform glandular arrangement, inflammatory cells within the tall columnal epithelium, cuboidal basal cells are intact and stromal (red arrow). Maize like gland folds with hyperchromatic nuclei, diagnosis: prostatitis – benign lesion. Plate 4 (Mag. X200) is a prostate tissue stained with periodic acid Schiff reagents (PAS). Section shows varied glandular arrangement, poorly differentiated glands, and a highly desmoplastic fibromuscular stroma; the glandular cells are very pleomorphic with hyperchromatic nuclei and prominent nucleoli (red arrow). The diagnosis is prostate cancer.
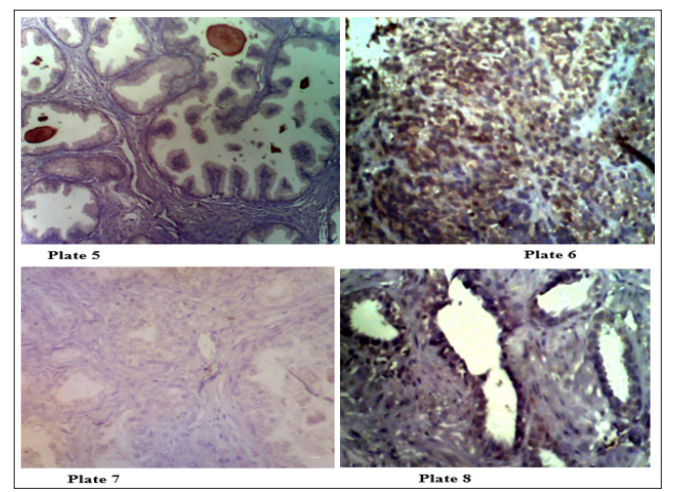
Plate 5 (Mag. X200) Immunohistochemical expression of AMACR is 1/3 Negative Corpora amylacea suggestive of BPH. Plate 6 (Mag. X200) Immunohistochemical expression of AMACR is 3/3 Positive for prostate adenocarcinoma. Plate 7 (Mag. X200) Immunohistochemical staining of prostate tissue section showing a weak expression (1/3) of CEA on benign prostate section. Plate 8 (Mag. X200) Immunohistochemical staining of prostate tissue section showing a strong expression (3/3) of CEA. This is positive for cancer of the prostate.
Discussion
Prostate cancer is one of the most diagnosed forms of cancer for men in the world, with an estimated 854,790 projected cases for 2021 [14]. Even more alarming is the observation that African men have a higher risk and a higher rate of prostate cancer morbidity and mortality compared to men of other racial or ethnic groups globally [15]. This present study reveals the immunohistochemical pattern and diagnostic applications of alpha-methyl CoA racemase and anti-carcinoembryonic antigen antibodies in prostate tumors. This demographic data from the sampled cases in this study shows that prostate cancer is most prevalent within the age group of 55–64 and least prevalent within the age group of 25–34.
This is in concordance with the report of Haas and Sakr who stated that the prevalence of prostate cancer increases with age, especially over the age of 60. The histological study of the prostate tissue section stained with H&E and PAS stains showed various histologic features indicative of both benign and malignant conditions of the prostate gland. H&E staining of some of the sections demonstrated well-differentiated columnar glandular epithelial cells, cuboidal basal cells, and concretions. These features are typical of benign prostate hyperplasia (BPH). This study also revealed inflammatory cells within columnar epithelium and gland folds with hyperchromatic nuclei, suggestive of prostatitis. Prostatitis is inflammation of the prostate gland, histologically characterized by infiltration of the prostatic ductus and periprostatic tissue, especially with polymorphic nuclear leukocytes. Perineural invasion and other features consistent with adenocarcinoma were observed. Perineural invasion is a phenomenon where prostate cancer cells invade and grow along prostatic nerves. Perineural space may be a microenvironment that fosters the growth and spread of cancer. Contrary to this present study, some studies did not find an association between perineural invasion and prostate cancer outcomes [16-20].
The stromal desmoplasia observed in this study is synonymous with cancer development. The outcome of the immunohistochemical demonstration of AMACR and CEA in this study shows the extent of expression of these markers. There is increasing evidence showing that AMACR is a useful marker for many cancers of the prostate, liver, kidney, and colon [21]. Sensitivity and specificity values for AMACR range from 82% to 100% and 79% to 100%, respectively [22]. In this study, 86.8% of the malignant tissue sections studied had a strong expression of AMACR. This corroborates the result of Kunju et al [23]. who noted that AMACR was overexpressed in prostate carcinoma cells when compared with benign or normal prostate epithelial cells. CEA is a glycosylated protein upregulated on the surface membrane of many types of cancer cells and commonly diagnostic marker for various cancers [24]. Overexpression is associated with many types of cancer, including gastrointestinal, respiratory, genitourinary, and breast cancers. Though CEA is one of the oldest and most widely used tumor markers for monitoring tumor recurrence after surgical resection and prognosis, its role in prostate cancer has not been clearly described [25]. This present study shows that these markers are useful in the diagnosis of prostate cancer.
Conclusion
The identification of immunohistochemical biomarkers capable of providing diagnostic and predictive insights on the course of cancer is paramount to tackling the problem of prostate cancer. In this study, AMACR and CEA have been demonstrated to be useful for the identification and evaluation of prostate cancer. The outcome of this study provides direction for the development of screening guidelines for early detection and monitoring of prostate cancer.
Ethics Approval
All processes were carried out in line with research-based ethical best practices for maintaining patients’ confidentiality as stated in the ethics committee board approval guidelines on human research.
Conflict of Interest
There are no conflicts of interest related to this study, according to the authors.
Funding
No grant was obtained for this study from a private or public institution.
Acknowledgement
We hereby acknowledge the understanding and compliance of our technical staff and Medical Laboratory scientists in Histopathology Laboratories for their assistance and support throughout the research.
References
- Yang D, Shi X, Lei Y, Zhou X, Chen Q (2020) The auxiliary diagnostic value of prostate-specific antigen and α-methylacyl- CoA racemase in prostate Oncol Lett 20: 1418-1422.
- Sharma S, Zapatero-Rodríguez J, O’Kennedy R (2017) Prostate cancer diagnostics: Clinical challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnol Adv 35: 135-149.
- Yang D, Shi X, Lei Y, Zhou X, Chen Q (2020) The auxiliary diagnostic value of prostate-specific antigen and α-methylacyl- CoA racemase in prostate Oncol Lett 20: 1418-1422.
- López Estebaránz JL, Zarco-Montejo P, Samaniego ML, García-Calvo C (2015) PREVAL Study Group. Prevalence and clinical features of psoriatic arthritis in psoriasis patients in Spain. Limitations of PASE as a screening tool. Eur J Dermatol EJD 25: 57-63.
- Jiang N, Zhu S, Chen J, Niu Y, Zhou L (2013) A-methylacyl- CoA racemase (AMACR) and prostate-cancer risk: a meta- analysis of 4,385 participants. PloS One 8: e74386.
- Hasan IA, Gaidan HA, Al-Kaabi MM (2020) Diagnostic Value of Cytokeratin 34 beta E12 (Ck34βE12) and α-Methylacyl- CoA racemase (AMACR) Immunohistochemical Expression in Prostatic Iran J Pathol 15: 232-238.
- Lázaro-Gorines R, Ruiz-de-la-Herrán J, Navarro R, Sanz L, Álvarez-Vallina L, et al. (2019) A novel Carcinoembryonic Antigen (CEA)-Targeted Trimeric Immunotoxin shows significantly enhanced Antitumor Activity in Human Colorectal Cancer Sci Rep 9: 11680.
- Taheri D, Roohani E, Izadpanahi MH, Dolatkhah S, Aghaaliakbari F, et (2021) Diagnostic utility of a-methylacyl COA racemase in prostate cancer of the Iranian population. J Res Med Sci Off J Isfahan Univ Med Sci 26: 46.
- Carneiro A, Barbosa ÁRG, Takemura LS, Kayano PP, Moran NKS, et (2018) The Role of Immunohistochemical Analysis as a Tool for the Diagnosis, Prognostic Evaluation and Treatment of Prostate Cancer: A Systematic Review of the Literature. Front Oncol 8: 377.
- Kahane H, Bostwick DG (2014) Florid Basal Cell Hyperplasia and Other Benign Mimics of Prostate Cancer. AJSP Rev Rep 19: 154-162.
- Okorie N (2022) Basic Histopathology Techniques: Instructional Manual for Staff and Students ISBN: 978-978-978-617-4.
- Yakubu GA (2016) Histopathological analysis of prostatic cancers in federal medical centre Fac Pathol [Internet] Available from: http://dissertation.npmcn.edu.ng/index.php/ FMCPath/article/view/1080
- Sharma S, Mudgal S, Thakur K, Gaur R (2019) How to calculate sample size for observational and experiential nursing research studies? Natl J Physiol Pharm Pharmacol 10: 1-9.
- Powell I (1998) Keynote address: prostate cancer among African-American men--from the bench to the community. J Natl Med Assoc 90: S705-S709.
- Powell IJ (1998) Prostate cancer in the African American: is this a different Semin Urol Oncol 16: 221-226.
- Haas GP, Sakr WA (1997) Epidemiology of prostate CA Cancer J Clin 47: 273-287.
- Nickel, Downey, Young, Boag (1999) Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int 84: 976-981.
- Zareba P, Flavin R, Isikbay M, Rider JR, Gerke TA, et al. (2017) Perineural Invasion and Risk of Lethal Prostate Cancer Epidemiol Biomarkers Prev 26: 719-726.
- Hashimoto T, Yoshioka K, Nagao G, Nakagami Y, Ohno Y, et (2015) Prediction of biochemical recurrence after robot- assisted radical prostatectomy: Analysis of 784 Japanese patients. Int J Urol 22: 188-193.
- Kozal S, Peyronnet B, Cattarino S, Seisen T, Comperat E, et (2015) Influence of pathological factors on oncological outcomes after robot-assisted radical prostatectomy for localized prostate cancer: Results of a prospective study. Urol Oncol Semin Orig Investig 33: e1-e7.
- Fadare O, Parkash V, Gwin K, Hanley KZ, Jarboe Elke A, et (2013) Utility of α-methylacyl-coenzyme-A racemase (p504s) immunohistochemistry in distinguishing endometrial clear cell carcinomas from serous and endometrioid carcinomas. Hum Pathol 44: 2814-2821.
- Ferdinandusse S, Denis S, IJlst L, Dacremont G, Waterham HR, et (2000) Subcellular localization and physiological role of α-methylacyl-CoA racemase. J Lipid Res 41: 1890-1896.
- Kunju LP, Chinnaiyan AM, Shah RB (2005) Comparison of monoclonal antibody (P504S) and polyclonal antibody to alpha methylacyl-CoA racemase (AMACR) in the work-up of prostate Histopathology 47: 587-596.
- Shinmi D, Nakano R, Mitamura K, Suzuki-Imaizumi M, Iwano J, et al. (2017) Novel anticarcinoembryonic antigen antibody–drug conjugate has antitumor activity in the existence of soluble Cancer Med 6: 798-808.
- Lee H, Kim M, Kim SH, Tran Q, Kong G, et al. (2020) Alpha-Methylacyl-CoA Racemase (AMACR), a Potential New Biomarker for Glioblastoma. Front Oncol [Internet]. Available from: https://www.frontiersin.org/articles/10.3389/ 2020.550673.

