Catheter-Related Infections in Hemodialysis Patients: A Retrospective Study
© 2023 Abdullah Hashim Almalki, Mariann Al-Jehani, Noor Alharbi, Afnan Malibari, Naji Dwid, et al., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Catheter-related infection (CRI) is a serious complication among hemodialysis patients, associated with significant morbidity and mortality. This study investigated the incidence, predictors, presentation, and complications of CRI.
Methods: This single-center, retrospective, observational study enrolled all eligible chronic hemodialysis patients with tunneled central venous catheters inserted between June 2016 and June 2019.
Results: Over 3 years, 63 patients (59% men) with a total of 27,395 catheter days were included of these patients. The median age was 68 years (interquartile range [IQR]: 58,76), and median hemodialysis duration was 62 months (IQR: 16,101). A total of 30 episodes of clinical CRI occurred, resulting in an overall incidence rate of 1.1 per 1,000 catheter days. CRI was significantly associated with baseline anemia (adjusted hazard ratio [AHR]=3.29; 95% confidence interval [CI], 1.42–7.64; P=0.006) and the use of the femoral vein as opposed to internal jugular vein (AHR=3.23; 95% CI, 1.29–8.06; P=0.012). The incidence of catheter-related bacteremia was 0.91 per 1,000 catheter days, and the most commonly isolated organism was Staphylococcus aureus (26%). Recurrent infection developed in 9/27 (33.3%) episodes and was lower among catheter salvage with antibiotic lock and catheter removal compared to catheter salvage alone.
Conclusions: Anemia and the use of the femoral instead of the internal jugular vein are associated with a higher incidence of CRI. Catheter-related blood stream infection was associated with increased mortality, recurrence rate, and resource utilization. Along with systemic antibiotics, catheter management such as catheter removal or antibiotic lock use may help reduce the recurrence rate
Introduction
End-stage renal disease (ESRD) is a major health problem associated with significant morbidity and mortality, part of which is related to hemodialysis (HD) access [1-3]. The Global Burden of Disease 2015 study estimated 1.2 million deaths worldwide resulting from kidney failure, which represents an increase of 32% since 2005 [4]. Infection is the second leading cause of death in HD patients and is a major cause of morbidity and hospitalization in this population. The majority of severe infections are caused by bacteremia, followed by pneumonia [5]. Vascular access is a major risk factor for bacteremia, with a much higher risk with central venous catheter (CVC) than with arteriovenous fistula (AVF) [6, 7]. AVF is known as the gold standard access for dialysis; however, high rates of CVC use are reported for both incident and prevalent HD patients [8].
Catheter-related blood stream infection (CRBSI) is one of the serious complications in HD patients using CVC [9]. Several international series after 2010 have reported a frequency of catheter-related bacteremia of less than two episodes per 1,000 catheter days [10-12]. which showed an improvement when compared to findings reported in the majority of publications before 2010 [13-15].
In Saudi Arabia, there is a paucity of published data regarding vascular access and related complications, particularly regarding the risk of infection and bacteremia. A single center study published in 2011 reported a 2–4 fold higher access-related bacteremia among Saudi HD patients when compared to the international benchmark from the US National Healthcare Safety Network centers [16].
There is a significant knowledge gap regarding catheter-related infection (CRI) in HD patients in Saudi Arabia. Therefore, this study aimed to examine the incidence, potential predictors, presentation, treatment, and clinical outcomes of CRI among HD patients.
Materials and Methods
In this single center retrospective study, we included all eligible chronic hemodialysis patients who underwent tunneled CVC insertion between June 1, 2016, and June 31, 2019. The study included adult patients aged >18 years who were on chronic hemodialysis with a tunneled CVC inserted at the same center and a minimum of 2 days of catheter life. Any patients with no access to follow-up data were excluded. In previous studies, the incidence of CRBSI was reported at 1.1–5.5 episodes per 1,000 catheter days [17-19]. Accordingly, to achieve an incidence rate with a 95% confidence level and a relative precision of 0.03, our study required data collected from at least 48 patients with an average of 90 catheter days to satisfy a minimum of 4,269 catheter days. The proposal was approved by the local institutional ethics committee (Study number: SP18/319/J). As this study comprised a retrospective chart review with no patient contact, per the institutional guidelines, informed consent was not required. The data collected included demographics, medical history, occurrence of CVC-related infection, potential predictors, clinical presentation, bacteriology, and treatment pattern with regard to catheter management (catheter exchange, removal, or salvage with the use of antibiotic lock). Among the potential predictors, the last laboratory data reported within 1 month prior to the onset of suspected blood stream infection (BSI) or the end of the study period was considered as baseline data. Outcome data included hospitalization, length of stay, 90-day mortality, and recurrence. CRI was defined either by (1) laboratory findings of any positive blood culture finding obtained from the CVC or peripheral vein without any other source of infection or (2) when physicians clinically establish the diagnosis and treatment for sepsis in patients with at least one clinical sign of fever or hypotension in the absence of other recognized causes [20]. The charts of all patients who received systemic antibiotics were identified, reviewed, and classified as CRI or infection from other sources. In this paper, we use the term CRI to include all symptomatic patients with and without laboratory confirmation of BSI. Diagnosis for culture-negative cases was verified through reviewing charts to rule out other sources of infection and/or to observe defervescence of symptoms after antibiotic treatment or catheter removal.
The data were analyzed using SPSS Statistics for Windows, version 26 (SPSS Inc., Chicago, Ill., USA). Descriptive statistics are presented as mean (standard deviation) or median (interquartile range [IQR]) for continuous data, as appropriate. Categorical data are presented as counts and proportions for baseline characteristics, catheter management, and 90-day follow-up data. Finally, the predictors of CRI were analyzed using time-to-event analysis, log rank, and the cox proportional hazard model. A p-value <0.05 was considered significant.
Results
From June 1, 2016, to June 31, 2019, 92 catheters were inserted a total of 63 patients, contributing to 27,395 catheter days. Of these patients, 37 (59%) were male, the median age was 68 years (IQR: 58,76), and the median duration on HD was 62 months (IQR: 16.4,100.8). Most catheters were inserted in the internal jugular vein (82.5%), and some patients had more than one catheter inserted at different time points during the study period. The patient and catheter characteristics are summarized in Table 1.
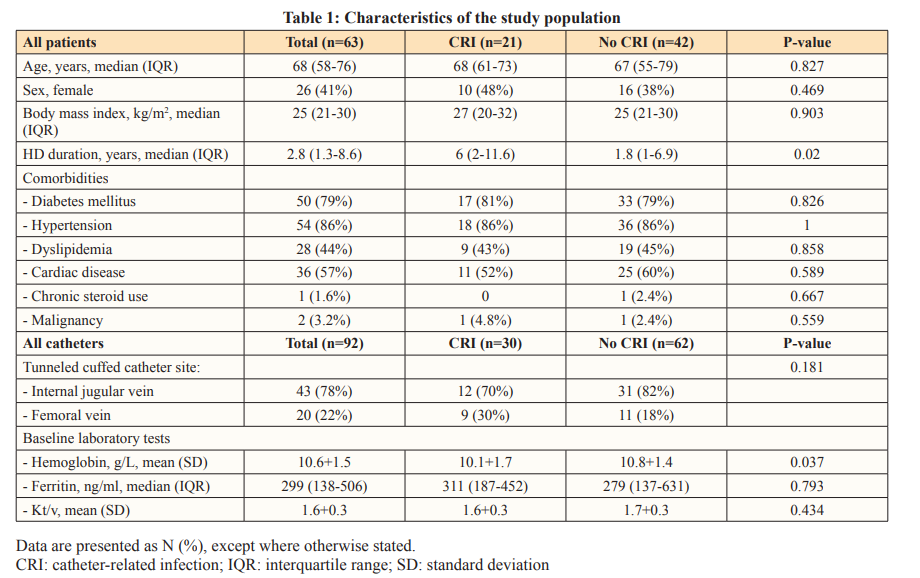
CRI Incidence Rate
Among the 63 patients, 30 episodes of CRI were noted for a total of 92 catheters, resulting in an overall incidence rate of 1.1 per 1,000 catheter days. The median time to CRI was 165 days (IQR: 93,308), and the incidence rate for bacteremia was 0.91 per 1,000 catheter days, with a median time to bacteremia of 191 days (IQR: 102,438).
Clinical Presentation and Bacteriology
The symptoms and signs of CRI included fever/chills (83%), hypotension (34%), altered mental status (21%), nausea/vomiting (21%), and septic shock (17%). Moreover, concomitant exit- site (ES) infection and/or tunnel infection were found in 18%. Leukocytosis was evident in 15/30 episodes (50%), with a mean white blood cell count of 10.2 (standard deviation [SD], 4.8), and C-reactive protein (CRP) was elevated in all 25 tested episodes (100%), with a median CRP of 64 mg/L (IQR, 26–160). These data are presented in Table 2.
The results of the blood cultures were positive in 23 (77%) episodes: 10 episodes involved gram-positive cocci (43%) and the remaining 13 involved gram-negative bacilli (57%). Among the positive findings for culture specimens, the most frequently isolated organisms were Staphylococcus aureus, Pseudomonas sp., and Staphylococcus epidermidis (CNSE), with 26%, 22%, and 13%, respectively. No methicillin-resistant Staphylococcus aureus was reported in this cohort, while two episodes were attributed to extended-spectrum beta lactamase (ESBL) organisms.
CRI Predictors
Multivariate analysis using the cox proportional hazard model was conducted to examine the potential predictors of CRI. Catheter site, anemia, ferritin level, and urea clearance index (Kt/v) were included in the model. Baseline anemia (hemoglobin<10 g/L) was associated with a higher risk of CRI (adjusted hazard ratio [AHR]=3.29; 95% confidence interval [CI], 1.42–7.64; p-value=0.006). The use of the femoral vein, as opposed to the internal jugular vein, was also associated with a higher risk (AHR=3.23; 95% CI, 1.29–8.06, p-value=0.012).
Antibiotic and Catheter Management
Treatment of patients who reported the 30 CRI episodes with empiric antibiotics included the combination of vancomycin and ceftazidime (43%), vancomycin alone (10%), ceftazidime alone (3%), or others (43%). Most of the “others” included vancomycin with meropenem. The mean (SD) antibiotic duration was 17 (9) days. Catheter treatment for the 30 episodes of CRI included: (a) salvage alone in 8 (27%), (b) salvage with antibiotic catheter lock in 7 (23%), (b) catheter exchange in 6 (20%), and (d) catheter removal in 9 (30%) patients.
Clinical Outcomes
Majority of episodes, 24/30 (80%), required hospitalization, and 4 episodes (13%) required intensive care unit (ICU) admission. The median length of hospital stay was 14 days (IQR: 6,21). Disseminated infection with para-spinal abscess was reported in one episode (3.3%). This is presented in Table 2.
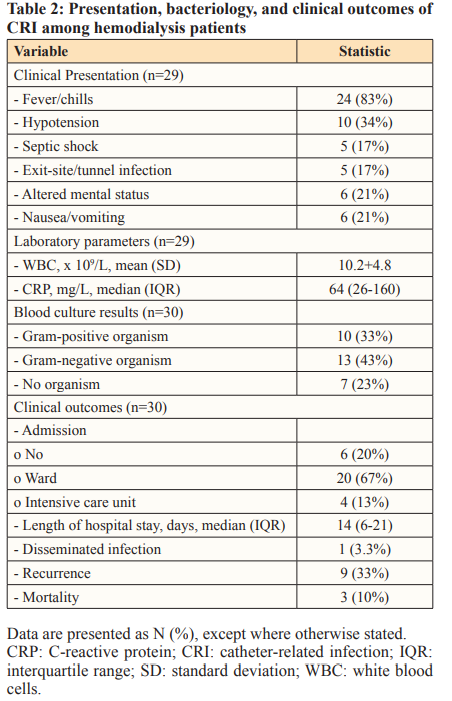
The recurrence of CRI developed in 9/27 survivors (33.3%; 95% CI, 14–52%), with a median time to recurrence of 34 days (IQR: 19,53). Numerically, the recurrence rate was observed to be higher among patients who underwent salvage without antibiotic lock (5/7) when compared to catheter removal (1/8) and salvage with antibiotic lock (0/7). When catheter exchange was utilized as an alternative option for catheter removal in 6 patients, recurrence developed in 3/5 patients. Of note, two patients had ESBL; of these, the catheter was managed with salvage alone in one patient and with catheter exchange in the other; however, both the patients developed recurrences.
A total of 10 patients died during the study period; of these, 3 were attributed to the first episode of CRI (10%). All three patients who died presented with hypotension and required hospitalization. One patient showed negative blood culture, one showed positive finding for coagulase-negative Staphylococcus epidermidis, and one showed positive finding for Klebsiella. Of these three deaths, one patient underwent catheter salvage alone, one underwent catheter exchange, and one underwent catheter removal.
Discussion
In this single-center study including 92 catheters of varying catheter days, we reported an incidence rate of clinical CRI of 1.1 and catheter-related bacteremia of 0.91 per 1,000 catheter days. Moreover, CRI was significantly more common among HD patients with baseline anemia (hemoglobin <10) and those with femoral catheters than among those with internal jugular catheters. In this cohort, gram-negative organisms were isolated more frequently (57%) when compared to gram-positive organisms; however, as a single organism, Staphylococcus aureus was the most frequently isolated organism (26%). Additionally, CRI was associated with a rate of hospitalization of 80% (ICU, 13%), septic shock in 17%, recurrence rate of 33%, and mortality in 3/30 episodes (10%). The recurrence rate was observed to be higher among patients who underwent salvage without antibiotic lock (5/7) when compared to catheter removal (1/8) and salvage with the use of antibiotic lock (0/7). Moreover, ESBL organism was identified in two episodes, and both developed recurrence with catheter management that did not involve catheter removal.
This study was conducted in a hospital HD unit. The institution serves two additional HD units as outsourcing units in the same region. Therefore, the more stable patients are usually referred to the outsourcing units, leaving the hospital unit with older and more comorbid HD patients. This may explain the higher rate of hypotension (35%), hospitalization (80%), and other complications in this cohort. Despite this, the overall incidence rate is comparable to recent local and international data. In particular, a similar rate was observed in a recently published local study reporting a CRBSI incidence of 1.4 episodes per 1,000 catheter days [16]. Several studies have reported an incidence rate ranging from 1.3–6.5 per 1,000 catheter days [13-15, 21, 22]. In contrast, the rate for bacteremia in our study was 0.91 per 1,000 catheter days. In a Danish study published in 2016, a similar rate of 0.9 per 1,000 catheter days was reported, with the use of an extensive prevention bundle approach [11]. A lower incidence of catheter- related bacteremia ranging from 0.19 to 0.63 per 1,000 catheter days was reported with the use of topical antibiotics from different studies [12, 18, 23].
Anemia was found to be a significant predictor for CRI (AHR=3.29; 95% CI, 1.42–7.64; p-value=0.006). A similar association was reported in other studies [24-26]. However, the mechanism behind the association of anemia with CRI is hard to explain. It was previously suggested that multiple blood transfusions or the administration of systemic iron to treat anemia may expose patients to iron overload, which increases the risk of infection due to the impaired phagocytic activity of neutrophils and increased chance of bacterial growth [27]. However, in our study, the baseline ferritin level was not significantly different between those who developed CRI and those who did not. Moreover, other risk factors such as older age, total intravenous iron dose, lower serum albumin level and diabetes mellitus were reported to have an association with the CRBSI risk in prior studies; however, none were found to have any association in our study, as well as in some other more recent findings from larger studies [10, 23].
The majority (77%) of our population with CRI had positive findings for blood cultures. Among the culture-positive episodes, we observed higher rates for gram-negative organisms than for gram-positive organisms (57% vs. 43%); however, Staphylococcus aureus remains the single-most commonly isolated organism. A similar observation of increasing gram-negative organisms causing bacteremia in HD was reported, approaching 40% or higher [16, 26, 28]. This can be attributed to poor compliance to infection control policies, a higher rate of diabetes mellitus or the misuse of antibiotics [16, 28, 29].
The overall recurrence of CRI in our study was reported in 30% of episodes and was more common for gram-negative organisms than for gram-positive organisms (33% vs 22%). This was also more common with catheter management that involved salvage alone or catheter exchange when used as alternative to catheter removal. Interestingly, the use of antibiotic lock in our study was associated with no recurrence. Similarly, catheter removal was associated with a lower rate of recurrence (1/8 [13%]). Therefore, our findings support catheter removal when indicated and the use of antibiotic lock when the catheter is not removed. However, the impact of catheter exchange as an alternative to antibiotic lock, i.e., when there are no indications for catheter removal, cannot be evaluated from our data. This is because catheter exchange was not routinely practiced in our center during the study period, owing to resource limitations related to interventional radiology. Therefore, the catheter exchange option was utilized as an alternative to catheter removal in patients with known difficulty in vascular access, as treating physicians may choose to use over-guidewire catheter exchange to avoid complete catheter removal and later “new insertion” that may carry the risk of failure to insert. Previous studies have shown lower rates of recurrent infection if CRBSI is treated with catheter exchange over a guidewire or with new replacement (catheter removal) when compared to salvage therapy [13, 15, 30].
We reported mortality due to CRI in three patients (10%). All were women and were sick with hypotension at presentation. Moreover, one patient showed culture-negative finding, one showed positive finding for coagulase-negative Staphylococcus epidermidis, and the third showed positive finding for Klebsiella organism. Of the three deaths, one patient underwent catheter salvage alone, one underwent catheter exchange, and one underwent catheter removal. Prior studies showed varying mortality rates of CRBSI, from 6–34%, and the mortality was particularly related to infective endocarditis [20, 31].
Our study has several limitations. First, the study had a retrospective single-center nature and small sample size. Second, the use of over- guidewire catheter exchange as an alternative to catheter removal makes it difficult to draw a conclusion on its efficacy to prevent recurrence in cases of CRI. It is possible that patients who require catheter removal have associated persistent ES or tunnel infection, which are best treated with catheter removal.
In conclusion, CRI developed at an incidence rate of 1.1 per 1,000 catheter days and was significantly associated with anemia and the use of the femoral vein as opposed to the internal jugular vein. More than half of the episodes in this cohort were associated with gram-negative organisms. In addition, our findings suggest a lower rate of recurrence for salvage therapy when used with antibiotic lock as compared to catheter salvage alone. Furthermore, when there are indications for catheter removal (e.g., in patients with septic shock, tunnel infection, or persistent infection), catheter removal, but not catheter exchange, is associated with a lower rate of recurrence. A future prospective study comparing catheter salvage with antibiotic lock and catheter exchange is therefore required to compare their efficacy in preventing recurrent infection.
References
- Lok CE, Foley R (2013) Vascular access morbidity and mortality: trends of the last decade. Clin J Am Soc Nephrol 8: 1213-1219.
- Celik S, Gok Oguz E, Ulusal Okyay G, Selen T, Ayli MD (2021) The impact of arteriovenous fistulas and tunneled cuffed venous catheters on morbidity and mortality in hemodialysis patients: A single center Int J Artif Organs 44: 229-236.
- Murt A, Yadigar S, Yalin SF, Dincer MT, Parmaksiz E, et (2021) Arteriovenous fistula as the vascular access contributes to better survival of hemodialysis patients with COVID-19 infection. J Vasc Access.
- GBD (2016) Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study Lancet 388: 1459-1544.
- Eleftheriadis T, Liakopoulos V, Leivaditis K, Antoniadi G, Stefanidis I (2011) Infections in hemodialysis: a concise review - Part 1: bacteremia and respiratory Hippokratia 15: 12-17.
- Pastan S, Soucie JM, McClellan WM (2002) Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620-626.
- Fysaraki M, Samonis G, Valachis A, Daphnis E, Karageorgopoulos DE, et (2013) Incidence, clinical, microbiological features and outcome of bloodstream infections in patients undergoing hemodialysis. Int J Med Sci 10: 1632-8.
- Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, et al. (2020) US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United Am J Kidney Dis 75(Suppl 1): A6-7.
- Allon M (2004) Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis 44: 779-791.
- Delistefani F, Wallbach M, Müller GA, Koziolek MJ, Grupp C (2019) Risk factors for catheter-related infections in patients receiving permanent dialysis BMC Nephrol 20: 199.
- Kaarup S, Olesen B, Pourarsalan M, Boesby L, Brandi L (2016) A retrospective quality study of hemodialysis catheter- related bacteremia in a Danish hospital. Open J Nephrol 6:
- Thompson S, Wiebe N, Klarenbach S, Pelletier R, Hemmelgarn BR (2017) Alberta Kidney Disease Network. Catheter-related blood stream infections in hemodialysis patients: a prospective cohort BMC Nephrol 18: 357.
- Beathard GA (1999) Management of bacteremia associated with tunneled-cuffed hemodialysis J Am Soc Nephrol 10: 1045-9.
- Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, et al. (1997) Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med 127: 275-280.
- Saad TF (1999) Bacteremia associated with tunneled, cuffed hemodialysis Am J Kidney Dis 34: 1114-24.
- El-Saed A, Sayyari A, Hejaili F, Sallah M, Dagunton N, et al. (2011) Higher access-associated bacteremia but less hospitalization among Saudi compared with US hemodialysis Semin Dial 24: 460-5.
- Weijmer MC, Van Den Dorpel MA, Van de Ven PJ, Ter Wee PM, Van Geelen JA, et al. (2005) CITRATE Study Group. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis J Am Soc Nephrol 16: 2769-77.
- Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW (2003) Conly Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol 14: 169-179.
- Lee T, Barker J, Allon M (2005) Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis 46: 501-8.
- Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, et (2016) Canadian Society of Nephrology Vascular Access Work Group. Hemodialysis tunneled catheter-related infections. Can J Kidney Health Dis 3: 2054358116669129.
- Mosquera DA, Gibson SP, Goldman MD (1992) Vascular access surgery: a 2-year study and comparison with the Nephrol Dial Transplant 7: 1111-5.
- Abdelsalam H, Mohamed M, Helal MA (2020) Longevity and infection free survival of permicath. QJM: An International Journal of Medicine 113: 013.
- Moore CL, Besarab A, Ajluni M, Soi V, Peterson EL, et al. (2014) Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol 9: 1232-9.
- Hoen B, Paul-Dauphin A, Hestin D, Kessler M (1998) EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 9: 869-876.
- Roberts TL, Obrador GT, St Peter WL, Pereira BJ, Collins AJ (2007) Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis Kidney Int 66: 2429-36.
- Nanyunja D, Chothia MY, Opio KC, Ocama P, Bwanga F, et (2022) Incidence, microbiological aspects and associated risk factors of catheter-related bloodstream infections in adults on chronic haemodialysis at a tertiary hospital in Uganda. IJID Reg 5: 72-78.
- Waterlot Y, Cantinieaux B, Hariga-Muller C, De Maertelaere- Laurent E, Vanherweghem JL, et (1985) Impaired phagocytic activity of neutrophils in patients receiving haemodialysis: the critical role of iron overload. Br Med J (Clin Res Ed) 291: 501-504.
- Mohsin B (2017) Pattern of causative micro-organisms in catheter related blood stream infections in dialysis patients: experience from Saudi J Ayub Med Coll Abbottabad 29: 635-640.
- Abdul Gafor AH, Cheong Ping P, Zainal Abidin AF, Saruddin MZ, Kah Yan N, et al. (2014) Antibiogram for haemodialysis catheter-related bloodstream Int J Nephrol 2014: 629459.
- Ashby DR, Power A, Singh S, Choi P, Taube DH, et (2009) Bacteremia associated with tunneled hemodialysis catheters: outcome after attempted salvage. Clin J Am Soc Nephrol 4: 1601-5.
- Lok CE, Mokrzycki MH (2011) Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587-598.

