Assessment of Inflammatory Markers in COVID-19 Vaccinated Individuals in Port Harcourt, Rivers State, Nigeria
© 2023 Onyemaechi, Uchechukwu, Obisike, Uchechukwu Achor, Nwachuku Edna Ogechi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To assess the level of some inflammatory markers in COVID-19 vaccinated individuals in Port Harcourt, Rivers State, Nigeria.
Study design: Case-controlled study.
Place and Duration of Study: Obio/Akpor, Phalga and PAMEL Laboratories and Diagnostics, Port Harcourt, between August 2021- October, 2022.
Methodology: A total of 148 subjects, resident in Rivers State that had received at least the first dose of any of the COVID-19 vaccines and 50 subjects that were not vaccinated with COVID-19 vaccines were recruited for the study. The sampling method employed a convenient simple randomized sampling method with a questionnaire distributed to all participants. Ten milliliters of whole blood were drawn from each subject into a plain bottle and allowed to clot. The serum was obtained , and used for the analysis of inflammatory markers using ELISA methods. The parameters analyzed included: tumor necrosis factor- alpha (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP). GraphPad Prism version 9.04 of Apple Macintosh HD Big Sur (version 11.0) was used for statistical analysis and variation among means of parameters were considered significant at p<0.05.
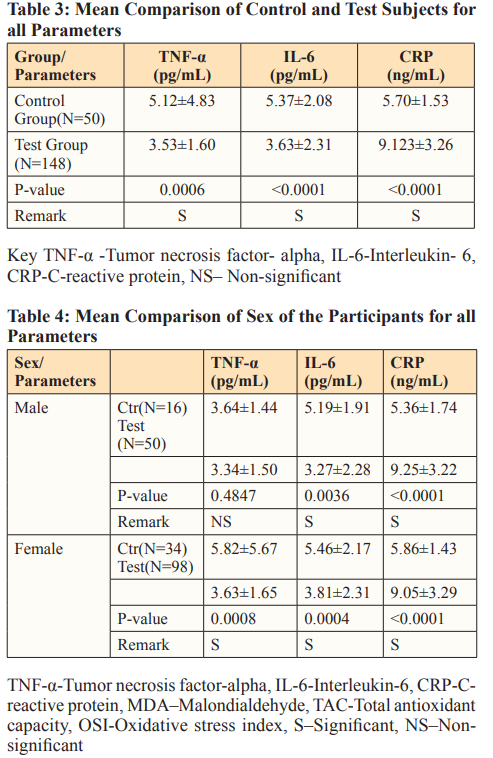
Results: There was significant decrease in IL-6 (p = 0.0006), TNF-α (p< 0.0001), and increase in CRP (p < 0.0001) among the vaccinated individuals when compared with the unvaccinated subjects. Comparison between the mean values at various age ranges 20-29, 30-39, 40-49, 50-59 showed significant higher values in IL-6 (p = 0.01076) among 30-39 age groups, there was no significant difference in TNF-α (p = 0.4341), CRP (p = 0. 5144). The comparison of the mean values of the parameters by sex among the two groups showed significant differences in IL-6 (p=0.0036) and in CRP (P<0.0001). There was significant increase in TNF-α (p = 0.0008), IL-6 (p = 0.0004), and CRP (p <0.0001) among female participants. IL-6 and CRP correlated positively with age.
Conclusion: The findings in this study suggest that COVID-19 vaccines may not be associated with inflammation. Also immunity built from the vaccine is not tied to the dosage (number of shots) or vaccine type.
Introduction
Sometime in late 2019 a new form of coronavirus emerged and brought with it a novel infection that affected people all over the world, the disease is known as coronavirus disease 2019, COVID-19 for short [1]. This infection is caused by sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with the first cases detected in China in December 2019 [2]. The virus infected so many people all over the world [3]. Over 33million people in United States were infected with over 3.1million deaths recorded nationwide [4]. The extent of the world widespread of the virus is not certain because of biases in test data. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19 is single stranded RNA+ virus that belongs to the genus of betacoronaviruses (BCoVs) which are a large group of viruses from the family of viruses called Coronavirida [5]. COVID-19 is among the novel coronaviruses which are responsible for causing acute respiratory tract infections (ARTIs) and is associated with pandemics that have affected humans. Other deadly pandemics that humans have experienced so far in the twenty-first century which are associated with anovel coronaviruses include SARS (severe acute respiratory syndrome) reported in China in the year 2002 and Middle East respiratory syndrome (MERS) reported in China in the year 2012. They are zoonotic viruses that spread to other species through bats/palm civets and dromedary camels, respectively [5]. All these viruses are highly contagious in nature and/or have caused high mortalities [6]. SARS-CoV-2 is highly transmittable than SARS-CoV-1and MERS-CoV.SARS-CoV-2 is also likely o have originated from bats, which have been serving as established reservoirs for various pathogenic coronaviruses. Although, it is still unknown how SARS-CoV-2 is transmitted to humans, but the rapid human-to-human transmission has been confirmed widely. The disease infects the respiratory tract producing flu-like symptoms, may cause severe illness like pneumonia and failure of multiple organs that can lead to death [7, 8]. The World Health Organization (WHO) has approved the use of several COVID-19 vaccines for the management of COVID -19 disease. The use of this vaccine is yet to gain global acceptance due to some suspected negative effects that could arise through its use. Some of the vaccines have been implicated in certain health disorders such as myocarditis and pericarditis [9]. There is theoretical risk that vaccination could make subsequent infection more severe [10]. Also, there could be internal alteration in the normal body makeup that could lead to future health issues, in other words, vaccine-associated enhanced disorders. In Nigeria, the rate of distribution and administration of COVID-19 vaccine is low due to high level of uncertainty of the safety of the vaccine [11]. Thus, the need to investigate the effect of these vaccines on inflammatory markers becomes imperative.
Some studies have shown that some Covid-19 vaccines can cause heart inflammation (Myocarditis) and create risk for future diseases due to vaccine-induce adverse effects. There seems to be no comprehensive safety data from the vaccine trials to improve the confidence on intake of the vaccine and mitigate the impression created in the minds of people about possible side effects that could arise from intake of COVID-19 vaccines. Nonetheless, there are speculations that the rapid production of these vaccines made them unsafe. This study will provide data on the inflammatory profile of COVID-19 vaccinated individuals in Port Harcourt, Rivers State, Nigeria.
Materials and Methods Study Area
The study area is shown on the map below (Figure 1)
Figure 2: Map of Rivers State Showing Port Harcourt Metropolis
Subjects Recruitment
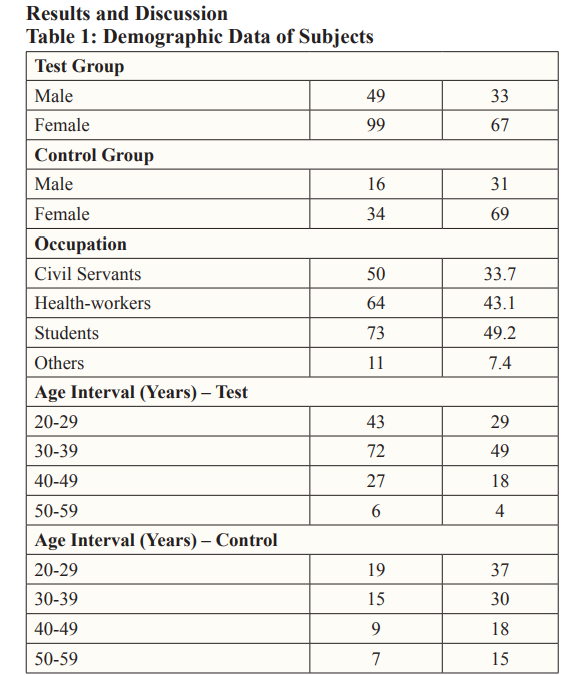
One hundred and ninety-eight (198) subjects, including male and female within the age of 20 – 59 years resident in Port Harcourt city (within Obio/Akpor and Phalga L.G.A) constituted the study population of this work. Among the one hundred and ninety-eight subjects recruited for this study were one hundred and forty- eight COVID-19 vaccinated individuals who constituted the test group while the remaining fifty are the control groups which are non COVID-19 vaccinated individuals. The study analyzed some inflammatory parameters which include (C-reactive protein, tumor necrosis factor -alpha and interleukin-6) in all the recruited subjects.
Study Design
This study is a case-controlled observational study that comprised one hundred forty-eight (148) subjects that were vaccinated with COVID-19 vaccine and fifty (50) control subjects that were not vaccinated with COVID-19 vaccines. The sampling method employs a convenient simple randomized sampling method.
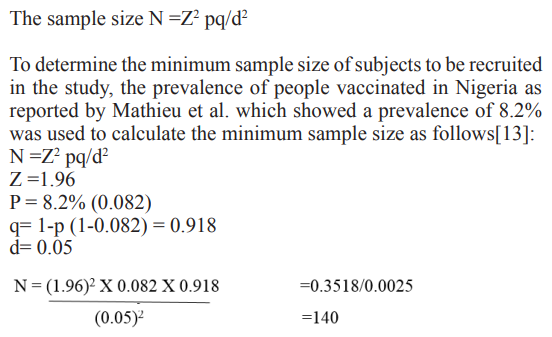
Sample Size Determination
Sample size was calculated using the formular as described by Charan & Biswas [12], with absolute error of 5% and 95% confidence interval.
Selection Criteria Inclusion Criteria
Subjects included in this work were those within the ages of 20 and above that gave their verbal consent and have taken any of the COVID-19 vaccines without history of health disorders such as cardiovascular disorders, liver and kidney dysfunction.
Exclusion Criteria
Those that consented to the study, up to age 20, and have taken at least one approved vaccine but have any clinical condition that could affect the results were excluded from the study.
Sample Collection, Processing and Analysis Sample Collection
Ten (10mls) of whole blood samples were collected from the participants into sterile plain bottles, properly labeled, separated, and the sera stored in the freezer till the day of analyses. The serum samples were transported in an ice pack to the laboratory.
Sample Processing
The blood samples were placed on the bench at 25°Cfor 40minutes to allow clotting of the blood before centrifugation. Clotted samples were retracted and spun for 10minutes at 3000rpm to obtain serum for examination of tumor necrosis factor-alpha(TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP). The serum samples were retrieved using Pasteur pippete into plain bottles and stored at -20°C in the freezer prior to analyses.
Sample Analysis
Determination of C-Reactive Protein, Tumor Necrosis Factor- alpha and Human Interleukin-6 (IL-6).
Method
This was done by indirect sandwich enzyme-linked immunosorbent assay (ELISA) quantitative assay method according to the Manufacturers Instruction (ElabScience.com).
Principle
The assay is based on Sandwish- ELISA principle. The ELISA plate well is sandwished with antibody specific to human for the analyte. Samples or standards when added to the micro-ELISA plate well combine with the specific antibody. On addition of a biotininylated detection antibody specific for the analyte, Avidin- Horserdish Perioxidase (HRP) conjugate and substrate reagent to the well an enzyme-substrate reaction is formed which will produce a blue coloration as product. After washing process, only those wells that contain the specific analyte, biotinylated detection antibody and avidin-HRP conjugate will retain the blue color. A stop solution is added to the reaction well to terminate the enzyme-substrate reaction to form a yellow-colored solution. The absorbance of the solution is measured spectrophotometrically at 450nm. The absorbance value obtained is proportional to the concentration of the analyte present in the sample or standard.
Statistical Analysis
GraphPad Prism version 9.04 for Apple Macintosh HD BigSur (version 11.0) was used for statistical analysis. Descriptive statistical tools such as mean & SD, Students independent sample t-test and ANOVA were used to compare means of two and more than two groups for inferential evaluation. Pearson ranked correlation was used to evaluate relationships between values in two groups. Bar and Pie charts were used to present demographic information. The probability (p) value less than 0.05 (p<0.05) was used and considered statistically significant.
Discussion
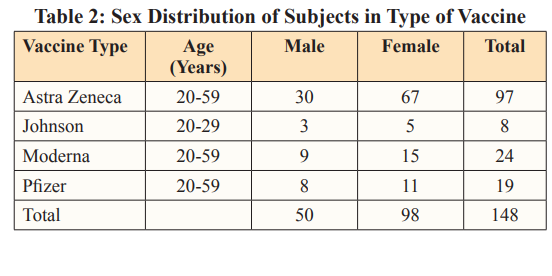
This study investigated the levels of some inflammatory markers in individuals (maleandfemale) that received COVID-19 vaccine. The demographic information on the prevalence of vaccine type showed that more people received AstraZeneca (66%) vaccine, followed by Moderna (16%), Pfizer(13%) and Johnson/Johnson (5%) (Table 1). The highest prevalence of vaccine intake seen in AstraZeneca could be as result of vaccine availability and the ease of its distribution to the country during vaccine deployment. AstraZeneca vaccine is cheap, according to Nike and Ebuka, [14]. The vaccine cost price is less than two bottles of beer, the vaccine is also easy to make and store (can be kept at normal refrigerator temperature of between two to eight degrees Celsius) making it suitable for developing countries like Nigeria [15]. Also, there is relative ease in sourcing for the ingredients used in the production of the vaccine as AstraZeneca has multiple supply and distributor chains which aid in easy deployment of the vaccine around the world. Also, AstraZeneca is thefirstCOVID-19 vaccine deployed in this part of the country which further suggests the reason for the highest prevalence in vaccine intake observed in this study.
In this study, the comparison between the mean values of tumor necrosis factor- alpha (TNF- α), C-reactive protein (CRP) and interleukin-6 (IL-6) among the test and control group showed statistically significant difference with the control groups having increased values in TNF-α andIL-6 as shown in Table3.Studies have shown that assessment of inflammatory markers including TNF-α, IL-6 and CRP is part of the recognized techniques for accessing and managing gCOVID-19 infection [16]. Although, these markers are not specific to COVID-19 and its vaccine. In this study, inflammatory markers (TNF-alpha and IL-6) were not increased in the vaccinated subjects ,which could be due to the mechanism of action of COVID-19 vaccines. Vaccines are known to mimic the actual disease, COVID-19 vaccine is not an exception to this phenomenon. In COVID-19, infected individuals present high levels of inflammatory cytokines resulting from cytokine storm but in COVID-19 vaccination there is temporary induction of inflammation as part of the mechanism of action of the vaccine in activating or initiating immune response. This inflammatory effect of thed vaccine is short term and does not cause harm as in real COVID-19 infection [17].
A study by Ahmed et al. revealed multisystem inflammatory syndrome as an adverse effect post COVID-19 vaccination in COVID-19 infected patients [18]. This study employed people that were not infected with COVID-19 prior to vaccination, this could be the reason for the inflammatory marker results obtained in this study. Also, strategies to neutralize inflammatory cytokine production and reduce inflammation are employed in the therapeutic and vaccination process against COVID-19 [19]. this implies that COVID-19 vaccines have the potential to attenuate inflammation and its effect in vaccinated individuals. This further suggests the reason inflammatory markers are not elevated in vaccinated individuals in this study, which implies that the temporary inflammatory actions induced by the presence of the vaccine in the vaccinated subjects would have been attenuated or waned.
The findings from this study revealed significant increase in CRP level among the vaccinated individuals (Table3), which is similar to a study by Ahmed and his colleagues that revealed acute inflammation with laboratory evident of elevated CRP in SARS- CoV-2 vaccinated individuals. [20]. The significant increase in CRP observed in vaccinated individuals could be as a result of the mechanism of action of the vaccine that includes temporary elevation of CRP, which eventually reduces when immune cells are fully recruited. CRP is one of the basement inflammatory markers raised in vaccination with COVID-19 vaccine, and serves as a representative biomarker to ascertain humoral immune response to vaccine and vaccine efficacy. Therefore, the significant increase in CRP level observed in vaccinated individuals suggests effective immunity and vaccine efficacy. The elevation of CRP levels in COVID- 19 vaccinated individuals is indicative of effective immunity.
This is in line with a study by Gian et al [21]. Which reveals a highly significant association between serum CRP concentration and anti- SARS- CoV-2 spike trimeric RBD IgG antibodies variation after COVID-19 vaccination. Combining this data from Gian and colleagues with our findings on CRP levels in COVID-19 vaccinated individuals, it could be hypothesized that the humoral response post COVID-19 vaccination may be proportional to baseline inflammation as reflected by the CRP level of the individuals. It is notable that there are other factors that can elevate CRP levels, such factor like in chronic disease state, in such situation the elevation of CRP is long-term but in case of vaccination with COVID-19 vaccine, the increase in CRP is part of immune activation process, which is usually short time, unlike the long-term elevation associated with those other health risks [17]. Also, In line with observation from this study, Sahin et al. 2020 in their study revealed elevation of CRP in individuals vaccinated with mRNA vaccines [22].
In this study, disparity in the concentration of the parameters was observed between female and male subjects with females having higher values in the concentration of the parameters analyzed, this could be due to modulation of internal body activities by both hormonal and genetic factors, in respect to sex (Table 4). Studies have revealed effects of estrogen (in females) and testosterone (in males), where there is speculation that higher concentrations of estrogen in female may aid heightened vaccine response in them, whereas, in males, testosterone has been linked to attenuated vaccine response [23,24]. According to Flanagan et al. females develop higher antibody responses and report more adverse response following vaccination, this is suggestive of the reason makers analyzed in this study are higher in females than in males. From this study, there was significant increase in IL-6 among females within the ages of 30-39 when compared against age group 40-49. This is in line with the study by Halsey et al. which revealed that in females hypersensitive reactions following vaccination were four times common in females than in males with increased ratio among individuals aged 30-39 [25]. In case of vaccination with COVID-19 vaccines, inflammatory markers which include IL-6 (one of the makers expressed during vaccination) have been observed to increase significantly at early time of vaccination.
There is possibility that time of sample collection is part of the reasons for the hike in IL-6 values seen among the age group 30-39. From this study, it was observed that people within age 20-40 received COVID-19 vaccine later than those of advanced age, this implies that the effect of the vaccine such as elevation of inflammatory markers is still viable as at the period of sample collection. Although, other factors could lead to the elevation of IL-6 level, making these associations non-specific.
When the levels of the parameters were considered in females for the various vaccine types taken, the results indicated no significant differences irrespective of the variation observed in the mean concentrations of the parameters analyzed. Also, the comparison of the levels of the parameters in male participants in respect to vaccine type taken did not show any significant difference for the various vaccine candidates, Findings in this study as shown in Table 3 showed significant increase in concentration of IL-6 and CRP in non-vaccinated male compared with their vaccinated counterparts. However, female control groups had significant increase in TNF-α than the male control group (Table4), this finding agrees with a study by Klein et al. which revealed that females express certain immune genes including gene for tumor necrosis factor which is higher in them than in males [26]. Also, study has it that at adulthood, inflammation is increased in female than in male and regardless of age females develop greater antibody responses and more adverse reaction following vaccination than males [26,27]. From this study, significant increase was observed in all the inflammatory markers among female control (unvaccinated) group when compared with their vaccinated counterpart (Table 4). The significant increase of inflammatory markers seen in control groups (both male and female) could be as a result of other inflammatory triggering factors that led to the release of inflammatory markers in them. General observation from this study revealed that women had higher concentration in the inflammatory markers analyzed, this is in line with the study by Klein et al. which revealed that females develop higher antibody responses and more adverse reaction following vaccination than males. Also, the high response to vaccine observed in female has been linked to female sex hormones [28, 29].
From this study there was on significant significant difference seen among the vaccinated and unvaccinated groups in respect to number of vaccine shots administered. This finding is in line with the actual principle of COVID-19 vaccine mechanism of action where there is notable reduction of effects induced by the presence of the vaccine in the body over time. Also, this finding is in line with a study by Kenji et al that revealed that immune function among vaccinated individuals eight months after the administration of two doses of COVID-19 vaccine was lower than that among the unvaccinated individuals [30]. A study by Choi et al. monitored immune responses of people who were initially vaccinated with two doses of mRNA-1273(Moderna) and who six months later received a third booster dose of multivalent vaccine containing both spike sequences and discovered that the boosters effectively restored the serum neutralization activity that had waned after the initial two-dose vaccination [31]. This suggests waning of the vaccine-induced immunity or effect over time. This study did not observe any significant association between the parameters and time passed since the vaccine was taken.
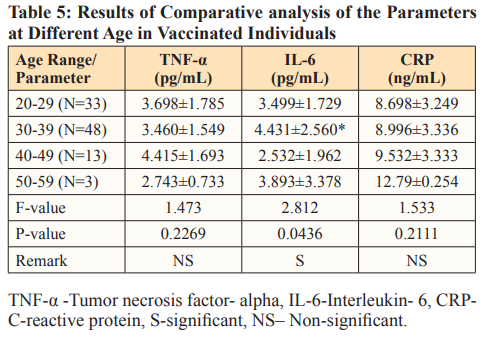
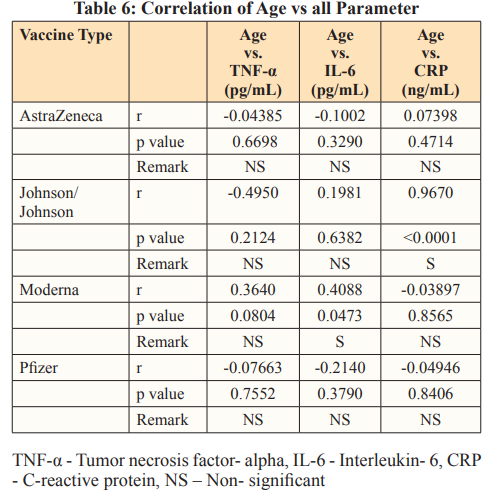
When the effect of age (Table5) on the parameters were considered as regards the various vaccine, significant positive association between age and CRP was observed in people vaccinated with Moderna, also a positive significant association was seen between age and IL-6 in subjects vaccinated with Johnson vaccine. Effects of age on pro-inflammatory makers have been established. The reason for the significant correlation results observed in this study could be because of natural aging and gender effect on inflammatory markers. Tables 6 and 7 further explain Table 5, where the relationship of age with inflammatory markers (especially IL-6) was established. This result is in tandem with that of a study by Juliana et al. where their major findings were a positive correlation between CRP and IL-6 as a function of age; women had highest values for CRP and IL-6 as also seen in our study [32].
Conclusion
From the study, significant increase in the values in some inflammatory cytokines were seen among the unvaccinated individuals. The vaccine type and number of shots have no effect on the parameters analyzed. Results obtained from the makers analyzed suggest that COVID-19 vaccines may not possess the potential of causing adverse inflammatory effects. Based on these findings, we could say that COVID-19 vaccines have the potential to attenuate inflammation in the body other than produce or cause such adverse effect.
AcknowledgEments
Authors wish to express their gratitude to Mr. Festus and Angela who assisted during sample collection; Sct. Njideka Chuks, Dr Elekima, Sct. Justina and all the staff of PAMEL Laboratories and Diagnostics for their assistance during the analytical stage of this study.
Competing Interests
Authors have declared that no competing interests exist.
Authors’ Contributions
Authors NEO and OUA designed and supervised the study, the latter performed the statistical analysis, wrote the protocol and the first draft of the manuscript, while author OU managed the laboratory analyses and literature searches. All authors read and approved the final manuscript.
Consent
Verbal consent was obtained from subjects before commencement of the study.
Ethical approval
Ethical approval was sought and obtained from the Rivers State Health Research Ethics Board of Rivers State Hospital Management Board through the Rivers State Ministry of Health. Nigeria,after explanation of the purpose of the study. A copy of the ethical approval letter could be provided on request.
References
- Health Organization (2022) Coronavirus disease (COVID-19) https://www.who.int/healthtopics/coronavirus#tab=tab_1.
- Kumar A, Kumar S, Narayan RK, Kumari C, Pareek V, et al. (2020) Expression of SARS-CoV-2 host cell entry factors in immune system components of healthy individuals and its relevance for COVID-19 immunopathology. Viral Immunology 34: 352-357.
- Toit DA (2020) Outbreak of a novel coronavirus. Nature Review Microbiology 18: 123-125.
- World Health Organization (2020) Coronavirus disease 2019 (COVID-19) Situation Report-68.
- Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic Nature Reviews Microbiology 17: 181-192.
- Tang YW, Schmitz JE, Persing DH, Stratton CW (2020) Laboratory diagnosis of COVID-19: Current issues and Journal of Clinical Microbiology 58: 512-520.
- Michael JP, Natarajan S, Emma AS, Daniela SC, Chima ON, et al. (2022) Mechanism of multi-organ injury in experimental COVID-19 and its inhibition by a small molecule peptide. Frontiers in Pharmacology 13:1-8.
- Van Eijk LE, Binkhorst M, Bourgonje AR, Offringa AK, Mulder DJ, et al. (2021) COVID-19: Immunopathology, pathophysiology mechanism, pathophysiological mechanisms, and treatment Journal of Pathology 254: 307-331.
- World Health Organization (2022) Coronavirus disease (COVID-19): Situation
- Meagan B, Eric D, Ralph B (2011) SARS-CoV and emergent coronaviruses: Viral determinants of interspecies Current Opinion in Virology 1: 624-634.
- Ryoko S (2022) COVID-19 vacccine hestitancy and trust in government in Vaccines 10: 1008-1011.
- Charan J, Biswas T (2013) How to calculate sample size for different study designs in medical research. Indian Journal of Psychological Medicine 35: 121-126.
- Mathew DS, Sourash S, Young HC, Veronique B, Soo KC, et al. (2013) COVID-19 vaccine development and potential nanomaterial path forward. Nature Nanotechnology 15: 646-
- Nike A, Ebuka O (2021) Analysis: Nigerian health experts debate concerns about AstraZeneca Premium Times News https://www.premiumtimesng.com/news/top-news/450319.
- Nike A (2020) COVID-19: Despite govt claims, experts say Nigeria not ready for vaccine distribution. Premium Times https://www.premiumtimesng.com/news/headlines/433281.
- Balta S, Balta I (2022) COVID-19 and Inflammatory Current Vascular Pharmacology 20: 326-332.
- Ioannis TP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, et (2022) Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends in molecular medicine 28: 542-554.
- Ahmad KU, Nidal MM, Mousa SA, Karim M (2021) Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 BMJ Case Reports 14: 242060.
- Meng QF, Tian R, Long H (2021) Capturing cytokines with advanced materials: A potential strategy to tackle COVID-19 cytokine Advanced Materials 33: 2100012.
- Ahmed M, Advani S, Moreira A, Zoretic S, Martinez J, et (2020) Multisystem inflammatory syndrome in children: A systematic review. Clinical Medicine 26: 100527.
- Gian LS, Brandon MH, Laura P, Simone DN, Giuseppe L, et
- (2022) reactive protein predicts humoral response after BNT162b2 booster administration. Journal of Infection 85: 24-25.
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, et al. (2020) COVID-19 vaccine BNT162b1 elicits human antibody and Th4 T cell Nature 586: 594-599.
- Trigunaite A, Dimo J, Jørgensen TN (2015) Suppressive effects of androgens on the immune Cellular. Immunology 294: 87-94.
- Flanagan KL, Fink AL, Plebanski M, Klein SL (2017) Sex and gender differences in the outcomes of vaccination over the life course 33: 577-599.
- Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, et (2013) Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (h4N1) vaccines: reports to VAERS. Vaccine 31: 6107-6112.
- Klein SL, Jedlick A, Pekosz A (2020) The Xs and Y of immune responses to viral vaccines. Lancet of Infectious Disease 10: 338- 349.
- Spitzer JA (1999) Gender differences in some host defense Lupus 8: 380-383.
- Klein SL, Marriot I, Fish EN (2015) Sex-based differences in immune function and responses to Transaction of the Royal Society of Tropical Medicine and Hygiene 109: 9-15.
- Hannah FM, Bajic VB Klein SL (2008) Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway Brain, Behaviour and Immunity 22: 503-516.
- Kenji Y (2022) Adverse effects of COVID-19 vaccines and measures to prevent Virology Journal 19: 1-3.
- Choi A, Koch M, Wu Kai W, Laurence C, LingZhi, et al. (2021) Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nature Medicine 27: 2025-2031.
- Juliana CM, Fernanda FA, Natalia MP, Vinicius M, Rehder- Santos, et al. (2019) Effects of natural aging and gender on pro-inflammatory markers. Brazilian Journal of Medical and Biological Research 52: 8392.

