Assessment of Antimicrobial Activities of Sida Acuta, Dioscorea Bulbifera and Citrus Aurantifolia Extracts on Selected Bacteria (Salmonella Typhimurium, Escherihia Coli 0157:H7 and Vibrio Cholerae)
© 2023 Amadi-Wali Owhorchukwu, Wokem Gloria Ngozika, Amala Smart Enoch, Azuonwu Obioma, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Medicinal plants are the primary source of medication used as complementary or alternative treatments to orthodox medicine. The assessment of the effects of plants, Dioscorea bulbifera (Air potato), Sida acuta (wired weed) and Citrus aurantifolia (Lime juice) extracts on selected bacteria (Escherichia coli0157:H7, Salmonella typhymurium and Vibrio cholerae) were investigated. The study area was Omuanwa in Ikwerre Local Government Area of Rivers State, Nigeria were the plants were collected. The test organisms were identified by polymerase chain reaction technique, while the antimicrobial effects of the plant extracts were examined against the different bacterial species by agar well diffusion method and sensitivity interpreted in accordance with the Clinical and Laboratory Standard Institute. Phytochemicals extracted were Flavonoids, Cardiac Glycosides, Tannin, Phenols, Alkaloids, Steroids, Terpenoid and Saponins, however, the concentrations of phytochemicals extracted by ethanol and aqueous showed statistical significant difference of p<0.05. The organisms were more sensitive to Citrus aurantifolia with 83.3%, followed by Sida acuta with 75.0% and Dioscorea bulbifera with 41.7%, also evident in the mean zones of inhibitions of the plant extracts, as the highest mean of 16.333mm was observed in Citrus aurantifolia against all test organisms followed by Sida acuta (15.083mm) and Dioscorea bulbifera (8.583mm). Escherichia coli0157:H7 was most sensitive to Sida acuta, Salmonella typhimurium was most sensitive to Citrus aurantifolia, while Vibrio cholerae showed equal-high sensitivity to Sida acuta and Citrus aurantifolia. Considering different percentage concentrations of plant extracts of 100%, 75%, 50% and 25% against the test organisms, the mean zone of inhibitions was in the order of increasing concentrations, the higher the concentrations the higher their zones of inhibition. The ability of the plant extracts to have shown cleared zone of inhibitions in this research showed the extracts to be potent antimicrobials against these test organisms
Introduction
Medicinal plants are the primary source of medication used as complementary or alternative treatments to orthodox medicine [1]. The act of nutraceutical, cosmeceutical and pharmaceutical industries using medicinal plants is gaining more attention around the globe. Dioscorea bulbifera. (Family: Dioscoreaceae) commonly known as ‘air potato’is an important edible medicinal plant use to treat a different range of diseases. Its traditional usage is also well known in the Chinese medicinal systems and also in West Africa [2]. The commercialization of medicinal plants as recognized in their uses by nutraceutical, cosmeceutical and pharmaceutical industries is gaining more popularity and interest around the globe. The plant family Amaryllidaceae has been known for a long time in the traditional medicinal system and used to treat varieties of diseases [2]. It is well known for its structurally-diverse alkaloids which exhibit a wide range of microbial activities. Past studies have also demonstrated that Dioscorea bulbifera is an active agent against several therapeutic diseases such as cancer, goiter, skin infections, pharyngitis and orchitis. Moreover, kidney and liver damage by this species is also reported by several researchers [2]. Dioscorea bulbifera has numerous medical applications owing to its inherent antibacterial, antifungal, plasmid curing, antidiabetic, antioxidant, and anticancer properties [2]. In the traditional settings, it has been used as a purgative, aphrodisiac, anthelmintic, rejuvenating tonic, diuretic, deflatulent and has been widely used for ameliorating scrofula, hemorrhoids, hematological disorders, diabetic disorders, polyurea, worm infestations, and skin diseases [3].
Citrus aurantifolia belongs to Rutaceae, it is a polyembroyonic plant cultivated in several part of the world especially hot subtropical or tropical region such as India, USA, Nigeria, Mexico. Lime crude extract have been reported to be very effective against varieties of microbes. They are well known for their antimicrobial properties against animal pathogenic bacteria.
Citrus aurantifolia (Limes) are a small citrus fruit it may either be sour or sweet; sour limes contains a greater sugar and citric acid content than lemons and feature an acidic and tart taste. The nutritional profile includes information on a full array of nutrients including carbohydrates, sugar, soluble and insoluble fiber, sodium, vitamins, minerals, fatty acids, amino acids and more [4]. Limes possess unique phytochemical compounds that have antioxidant and anti-cancer properties. While these compounds have been shown to inhibit cell division in many cancer cell lines. They are probably the most interesting for their antibiotic effects.
Sida acuta is family of Malvaceae which is an erect perennial herb and have branches, it is small and have small shrub that grows in large quantities on cultivated fields, waste areas, road sides and open clearing in Nigeria. Sida acuta shrub that originated from the pan tropical regions that are comprehensively spread and made use of in traditional medicine. The aerial segment of the plant is the most generally used. In Central America, the plant is mostly used for the therapy of asthma, kidney inflammation, colds, fever, headache, ulcers, and worms. The herb had in the olden times been used to cure malaria, diarrhoea, and different types of other illnesses [5]. Antimicrobial screening of Sida acuta indicated that numerous substances may be responsible for the plant’s action. The researchers noted that the plant’s methanolic extract had significant activity against varieties of microorganisms [5].
This study is to find out the antimicrobial effect of Sida acuta, Dioscorea bulbifera and Citrus aurantifolia extracts on Salmonella typhimurium, Vibrio cholerae and Escherichia coli0157:H7 test organisms, which are basically/primarily causes of gastroenteritis.
Aim of the Study
The aim of the study is to investigate the Assessment of the antimicrobial activities of Sida acuta, Dioscorea bulbifera and Citrus aurantifolia Extracts on Selected Bacteria (Salmonella typhimurium, Escherihia coli 0157:H7 and Vibrio cholerae).
Objectives of the Study
- To determine the phytochemistry of the plant extracts
- To Identify the test organisms by molecular technique (PCR)
- To determine the antimicrobial effect of Sida acuta extract on selected organisms (Salmonella typhimurium, Escherihia coli 0157:H7 and Vibrio cholerae).
- To determine the antimicrobial effect of Dioscorea bulbifera extract on selected organisms (Salmonella typhimurium, Escherihia coli 0157:H7 and Vibrio cholerae).
- To determine the antimicrobial effect of Citrus aurantifolia extract on selected organisms (Salmonella typhimurium, Escherihia coli 0157:H7 and Vibrio cholerae).
Research Questions of the Study
The following research questions were formulated to guide the study.
- What is the antimicrobial effect of Sida acuta extract on selected organisms (Salmonella typhimurium, Escherichia coli 0157:H7 and Vibrio cholerae)?
- What is the antimicrobial effect of Dioscorea bulbifera extracts on selected organisms (Salmonella typhimurium, Escherichia coli 0157:H7 and Vibrio cholerae)?
- What is the antimicrobial effect of Citrus aurantifolia extracts on selected organisms (Salmonella typhimurium, Escherichia coli 0157:H7 and Vibrio cholerae)?
Materials and Methods Study area
The study area for plant sampling is Omuanwa Community in Ikwerre local Government Area of Rivers State, it is situated between latitude 4054’0’’N and Longitude 6045’0’’E and 5012’0’’N and 703’0’’E (Figure 3.1). The mean annual temperature of the area is 280C. It is predominantly under the influence of the monsoon wind and also record heavy rainfall of 2370.5mm [6]. Generally, Rivers State is characterized by four biodiversity important vegetation zones, namely, the lowland rainforests, freshwater swamp forests, mangrove forests, and barrier island forests while Ikwerre local Government Area has freshwater swamp forests [6]. The Local Government Area is located in the Northern part of Rivers State Nigeria. Rivers State is located on the southern part of Nigeria bounded on the South by the Atlantic Ocean, to the North by Imo, Abia and Anambra States, to the East by Akwa Ibom State and to the West by Bayelsa and Delta States [7].
Experimental Design
The study adopted a 3 x 3 (3 by 3) factorial experimental design. This supports the use of three treatments (Sida acuta, Dioscorea bulbifera and Citrus aurantifolia) and three test organisms (Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae) for the study.
Plants Sample Collection
Fresh samples of Sida acuta leaves, Dioscorea bulbifera bulbs and Citrus aurantifolia fruits were collected from different farms in Omuanwa Community, Ikwerre Local Government Area of Rivers State. Thus were identified in the Department of Plant Science and Biotechnology, Faculty of Science, Rivers State University, Nkpolu-Oroworukwo, Port Harcourt.
Plants Extraction and Phytochemical Analysis
The plants samples (Dioscorea bulbifera and Sida acuta) were collected, washed with distilled water and dried at room temperature, blended and put in a sterile container for extraction. While fresh Citrus aurantifolia were washed with distilled water about three times and sterilized with 70% ethanol using spray bottle and was ready for extraction.
The extraction for Dioscorea bulbifera and Sida acuta was done using soxlet extractor for continuous extraction processes and batch extraction method with ethanol and distilled water [8]. A weighed portion of the pulverized sample (200g) was soaked in 1000ml of absolute ethanol and distilled water (BDHChemicals) for 72hours. The supernatant were decanted and filtered into a 1000ml conical flask through a N0. 1 Whatman filte paper for batch extraction while the soxlet extractor product was sent to the rotary evaporator. The extracts were concentrated using rotary evaporator set at 600C. While 250 ml pure juices of Citrus aurantifolia was collected and filtered with N0. 1 whatman filter paper.
It was then prepared in accordance with ISO17025. The following phytochemicals: flavonoids, cardiac glycoside, tannin, phenols, alkaloids, steroids, terpernoids and saponins were determined using the UV visible via scan analysis with the wavelength range of 200-1100nm. At each wavelength, its adsorption was compared with the UV developed standard for phytochemicals to determine the phytochemicals Present and it quantification was done with the help of the dilution factor which gives us the actual concentration. Then the values were subjected to statistical analysis using SPSS.
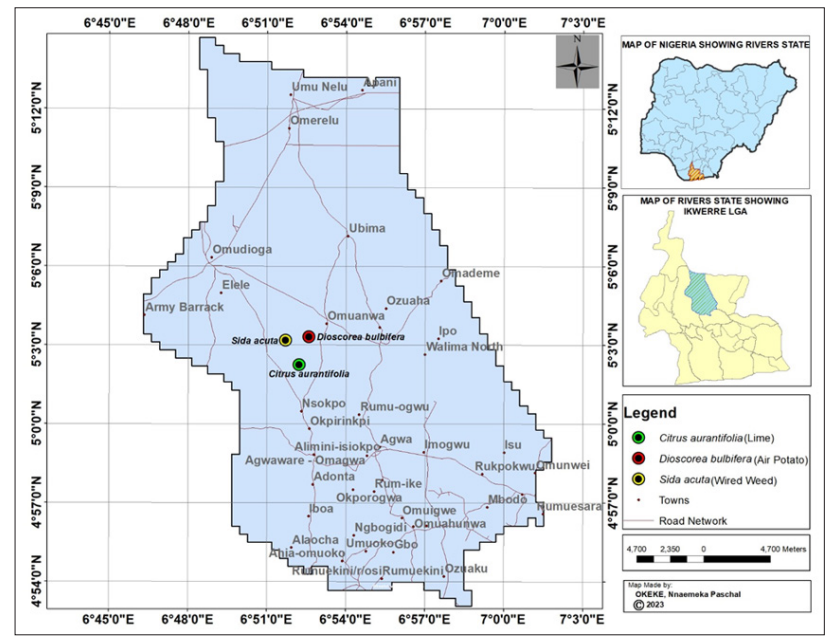
Map of the Study Area Showing Plants Sample Points
Collection of Test Bacteria (Escherichia Coli0157:H7, Salmonella typhimurium and Vibrio cholerae).
Salmonella enteric sub specie, enteric serova typhimurium derived from ATCC 14028 with lot number 363-543-5, expiry date of 31/01/2023 and E.coli 0157:H7 derived from ATCC 43888, lot number 795-209-2, expiry date 31/12/2022 were produced, packaged and shipped by Microbiologics 200 Cooper Avenue North, St. Cloud MN 56303, United States (www.microbiologics.com) while Vibrio cholerae (coded: VCP004/22) of a male 7years old was collected from Rivers State University Teaching Hospital of an Epidemiology reference laboratory for isolation of Vibrio cholerae.
Cultivation of the Test Organisms
The bacteria (Salmonella typhimurium and Escherichia coli0157:H7) came in KWIK STIK form which has the same instruction for use or preparations as follows.
The culture plates used to culture Escherichia coli0157:H7 were Nutrient Agar, MacConkey agar, Eosin Methylene Blue (EMB) and Cysteine Lactose Electrolyte Deficient (CLED) Agar. The ones used to culture Salmonella typhimurium were Nutrient Agar, Salmonella-Shigella Agar (SSA) and Deoxycholate Citrate Agar (DCA) while the one used to culture Vibrio cholerae are Nutrient Agar and Thiosulphate Citrate Bile Salt (TCBS) Agar.
Molecular Identification
DNA Extraction (Boiling Method)
Five (5) milliliters of an overnight broth culture of the bacterial isolate in Luria Bertani (LB) was spun at 14000rpm for 3 min. The cells were re-suspended in 500ul of normal saline and heated at 950C for 20 min. The heated bacterial suspension was cooled on ice and spun for 3 min at 14000rpm. The supernatant containing the DNA was transferred to a 1.5ml microcentrifuge tube and stored at -20oC for other downstream reactions.
DNA Quantification
The extracted genomic DNA was quantified using the Nanodrop 1000 spectrophotometer. The software of the equipment was lunched by double clicking on the Nanodrop icon. The equipment was initialized with 2 ul of sterile distilled water and blanked using normal saline. Two microliter of the extracted DNA was loaded onto the lower pedestal, the upper pedestal was brought down to contact the extracted DNA on the lower pedestal. The DNA concentration was measured by clicking on the “measure” button.
16s rRNA Amplification
The 16s rRNA region of the rRNA gene of the isolates were amplified using the 27F: 5’-AGAGTTTGATCMTGGCTCAG-3’ and 1492R: 5’-CGGTTACCTTGTTACGACTT-3’ primers on a ABI 9700 Applied Biosystems thermal cycler at a final volume of 40 microlitres for 35 cycles. The PCR mix included: the X2 Dream taq Master mix supplied by Inqaba, South Africa (taq polymerase, DNTPs, MgCl), the primers at a concentration of 0.5uM and the extracted DNA as template. The PCR conditions were as follows: Initial denaturation, 95ºC for 5 minutes; denaturation, 95ºC for 30 seconds; annealing, 52ºC for 30 seconds; extension, 72ºC for 30 seconds for 35 cycles and final extention, 72ºC for 5 minutes. The product was resolved on a 1% agarose gel at 130V for 30 minutes and visualized on a blue light transilluminator.
Sequencing
Sequencing was done using the BigDye Terminator kit on a 3510 ABI sequencer by Inqaba Biotechnological, Pretoria South Africa. The sequencing was done at a final volume of 10ul, the components included 0.25ul BigDye® terminator v1.1/v3.1, 2.25ul of 5 x BigDye sequencing buffer, 10uM Primer PCR primer, and 2-10ng PCR template per 100bp. The sequencing conditions were as follows 32 cycles of 96°C for 10s, 55°C for 5s and 60°C for 4min.
Phylogenetic Analysis
Obtained sequences were edited using the bioinformatics algorithm Trace edit, similar sequences were downloaded from the National Center for Biotechnology Information (NCBI) data base using BLASTN. These sequences were aligned using MAFFT. The evolutionary history was inferred using the Neighbour-Joining method in MEGA 6.0. The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analysed. The evolutionary distances were computed using the Jukes-Cantor method [9-11].
Sterility Test for The Plant Extracts
Each of the Plant Extracts of Sida acuta, Dioscorea bulbifera (aqueous and ethanol) and Citrus aurantifolia respectively were tested for sterility by culture before use. A 1g/ml of each of the extracts was plated by spread plate method with the use of a sterilized hockey stick on a freshly prepared nutrient media plates and incubated at 370C for 24hours. The plates were observed for growth [12].
Qualitative Screening and Determination of Antibacterial Activity
Agar Well
The screening of the antibacterial activities of the plants’ extracts and four conventional antibiotics were carried out for the determination of zone of inhibition by agar well as described by the National Committee of Clinical Laboratory Standards [13]. Clinical and Laboratory Standard Institute for antimicrobial disc susceptibility tests. Sterile Mueller-Hinton Agar plates were inoculated with already prepared inoculums of the different test organisms with a sterile cotton swab [14, 15]. Then with 6mm diameter sterile cork borer, different wells were made in the inoculated media plates. A sterile micropipette was used to transfer 50µl of the working solution or suspension of the different concentrations into the well. A control normal saline was also placed in the separate well at the same time and incubated at 370C for 24hours.
Statistical Analysis
The results of the quantitative analysis of phytochemical contents were presented as t-test. The frequencies distribution of agar well antimicrobial activities and the different concentration of extracts were all analyzed using percentages and analysis of variance (ANOVA). However, the statistical tool IBM SPSS statistics version 22 was used.
Results
Results in table.1 showed the comparison between the aqueous and ethanolic quantitative phytochemicals analysis of Sida acuta extract. Flavonoids, Phenols, Alkaloids, and Steroids extracted by the both solvents, however, Cardiac glycosides, Tannin and Saponins were extracted only by ethanolic while terpenoid was extracted by aqueous only. The mean concentration of phytochemicals extracted by the ethanolic solvent were higher than that of the aqueous solvent. Tannin had the highest ethanolic mean concentration of 50.00 while saponin had the least mean concentration of 0.28. In aqueous extraction, flavonoids had the highest mean concentration of 1.48 and alkaloids had the least mean concentration of 0.25. But anthroquinones and phobatannins were not extracted by the solvent. In the comparison, p-values (0.00) were significant for flavonoids, cardiac glycosides, tannin, phenols, steroids, terpenoid, and saponins, but not significant for alkaloids as p-value (0.419) is greater than 0.05.
Results in table.2 represent the comparison between the aqueous and ethanolic quantitative phytochemicals analysis of Dioscorea bulbifera extract. Flavonoids, Tannin, Phenols, and Alkaloids extracted by the both solvents, however, Cardiac glycosides, and Saponins extracted only by ethanol. In the comparison, p-values (0.00) are significant for flavonoids, cardiac glycosides, tannin, phenols, terpenoid, and saponins, but not significant for alkaloids as the p-value (0.1835) is greater than 0.05. Some of the phytochemicals such as steroid, anthroquinones, terpernoids and phobatanins not extracted by the solvents. Phenols had the highest ethanolic concentration of 18.40 while alkaloids had the least concentration of 0.68. In aqueous extraction, flavonoids had the highest concentration of 6.68 and tannin had the least concentration of 0.07.
Results in table. 3 of the quantitative phytochemicals analysis of Citrus aurantifolia extract, shows that the following phytochemicals: Flavonoids, Tannin, Phenols, and Alkaloids, Steroids, Terpenoid and Saponins were extracted. Alkaloids had the highest concentration of 84.00 while terpenoids had the least concentration of 0.53. No solvent was used for the extraction because lime juice is already in the liquid state.
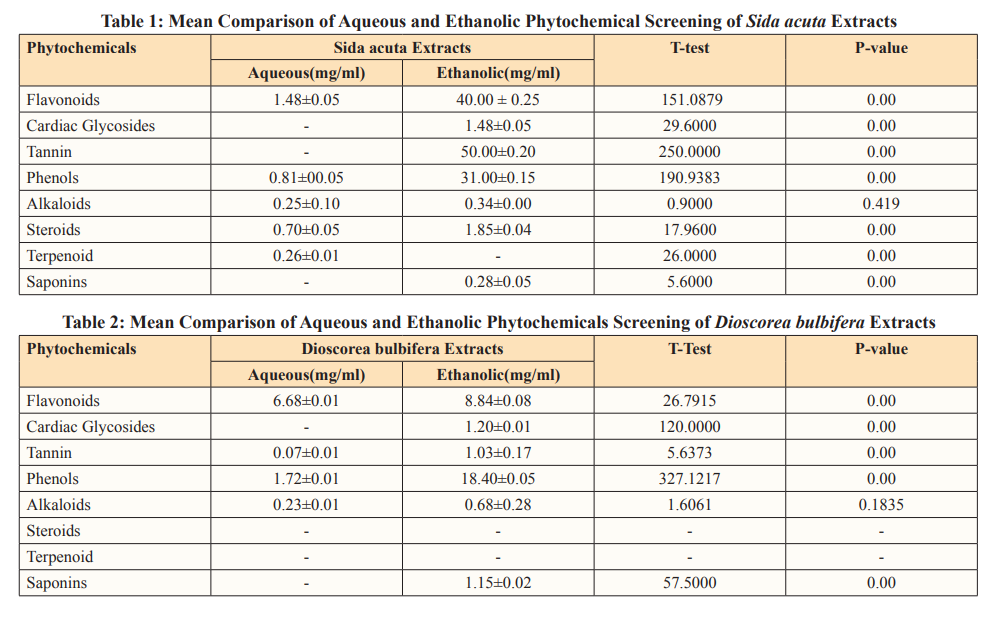
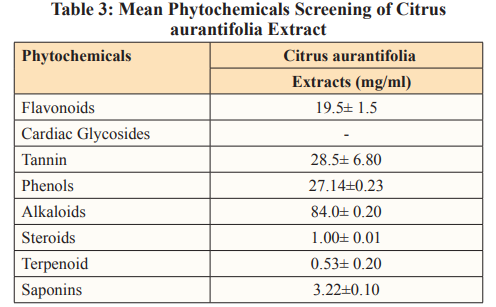
Figure 1 represents the results of the agarose gel electrophoresis of the selected bacterial isolates. Lanes 1 – 3 represent 16SrRNA gene bands (1500bp) of Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae respectively. Lane L represents the 100bp Molecular ladder indicated at 500bp.
In Figure 2 and 3, the obtained 16s rRNA sequence from the isolate produced an exact match during the megablast search for highly similar sequences from the National Center for Biotechnology Information (NCBI) non-redundant nucleotide (nr/nt) database. The 16S rRNA of the isolate M1 showed a percentage similarity to other species at 100%. The evolutionary distances computed using the Jukes-Cantor method were in agreement with the phylogenetic placement of the 16S rRNA of the isolates A1, A2 and VC within the Escherichia, Salmonella, and Vibrio spp. and revealed a closely relatedness to Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae respectively.
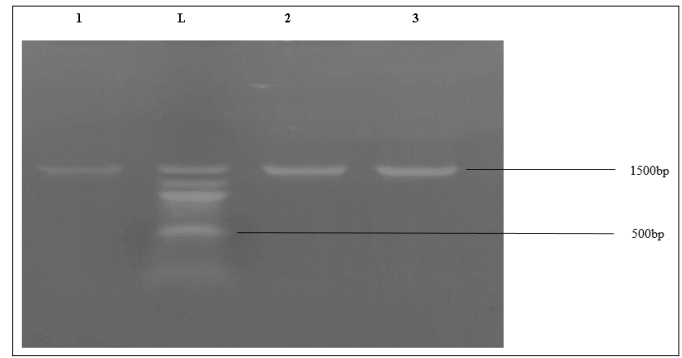
Figure 1: Agarose Gel Electrophoresis of Test Organisms
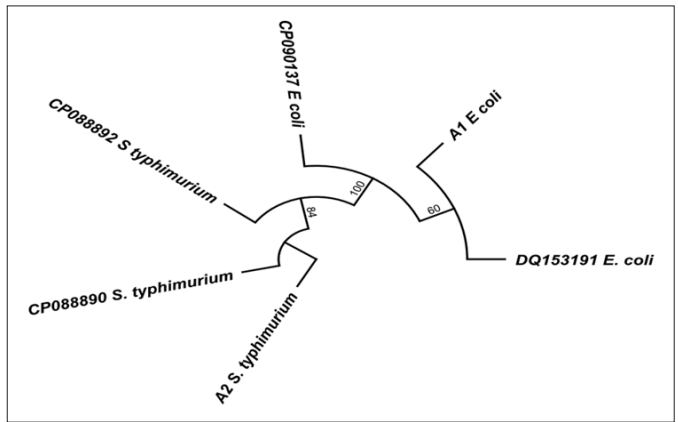
Figure 2: Phylogenetic Tree Showing the Evolutionary Distance Between the Bacterial Isolates of Escherichia coli0157:H7 and Salmonella Typhymurium
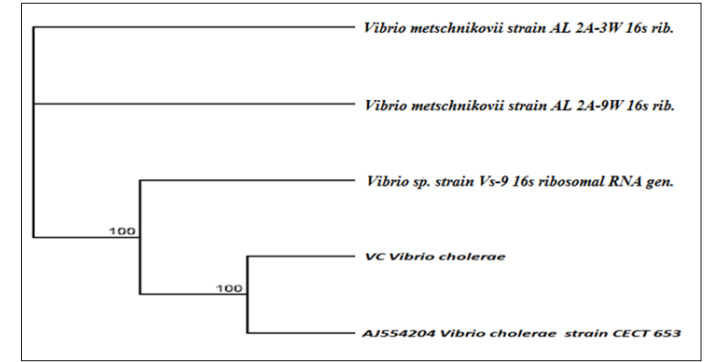
Figure 3: Phylogenetic Tree Showing the Evolutionary Distance Between the Bacterial Isolates of Vibrio cholerae
Table 4. shows the frequency distributions of the antibiogram (extracts) based on the percentage sensitivity and resistance irrespective of the concentrations. Amongst the single/mono therapy, the organisms were more sensitive to Citrus aurantifolia with 83.3%, followed by Sida acuta with 75.0% and Dioscorea bulbifera with 41.7%. On the other hand, Dioscorea bulbifera showed the highest level of resistance with 58.3% followed by Sida acuta with 25% and finally Citrus aurantifolia with 16.7%.
Table 5. presents percentage distributions of the antimicrobial activity of each extracts on the test microorganisms based on their percentage sensitivity and resistance. Amongst the single extracts, Escherichia coli0157:H7 was 100.% sensitive and 0% resistance to Sida acuta, 75% sensitive and 25% resistance to Citrus aurantifolia and 25% sensitive and 75% resistance to Dioscorea bulbifera. Salmonella typhimurium showed 100% sensitive and 0% resistance to Citrus aurantifolia, 50% sensitive and 50% resistance to Sida acuta and Dioscorea bulbifera. Whereas, Vibrio cholerae showed 75% sensitive and 25% resistance to Sida acuta and Citrus aurantifolia, then 50% sensitive and 50% resistance to Dioscorea bulbifera.
Table 6. describes the overall mean antibiogram activity (zone of inhibitions) of extracts, among the single category of the extracts, Citrus aurantifolia showed the highest mean of 16.333(mm), followed by Sida acuta 15.083(mm) and Dioscorea bulbifera with the least mean of 8.583(mm).

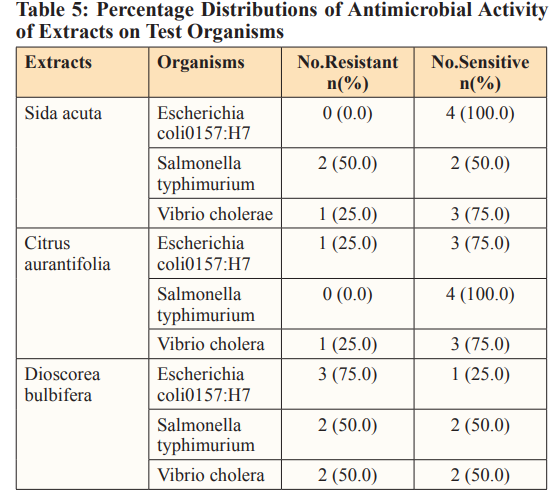
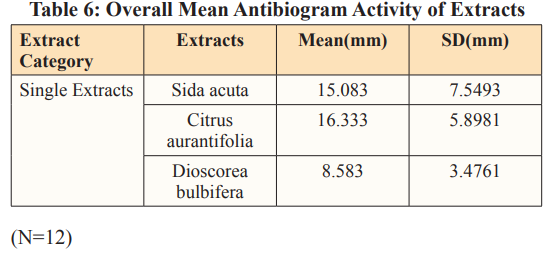
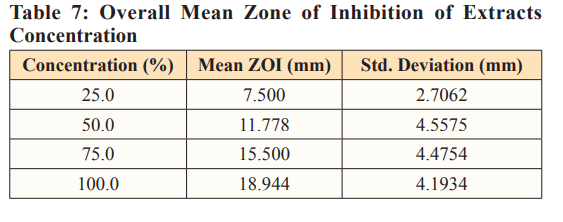
Table 7. demonstrates the overall mean zone of inhibition of different concentrations of extracts, ranging from the single extracts to the combine extracts against Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae. The mean zone of inhibitions is in the following order of increasing concentrations; 25.0 (7.500mm), 50.0 (11.778mm), 75.0 (15.500mm) and 100.0(18.944mm). Finding implies that the higher the concentration, the higher the zone of inhibitions.
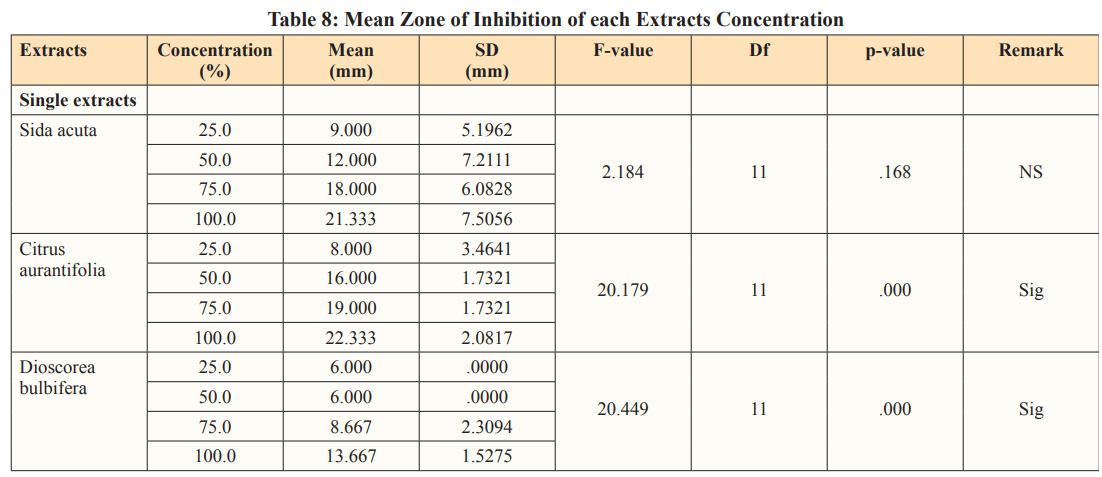
The table 8. below describes the mean zone of inhibition of each extracts concentrations, which indicates that there was statistical significance difference in Citrus aurantifolia (F=20.179, df=11, p=0.00) and Dioscorea bulbifera (F=20.449, df=11, p=0.00) except the Sida acuta (F=2.184, df=11, p=.168) of the single extracts.
Discussion
One of the major objectives of this research was to find out the antimicrobial activities of Dioscorea bulbifera, Sida acuta and Citrus aurantifolia on selected microorganisms (Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae). This was achieved by ascertaining the phytochemical analysis and antimicrobial activities of the plant extracts by the evaluation of the zones of inhibitions of the plant extracts. The reason for this investigation is to curb the random consumption of these extracts in speculation for the management of stomach disorders without specifications, as these tests organisms are most of the causes of gastroenteritis. The results obtained were analysed statistically and compared with standard limits.
Results of phytocemical analysis carried out on the different plant extracts showed different secondary metabolites such as Flavonoids, Cardiac Glycosides, Tannin, Phenols, Alkaloids, Steroids, Terpenoid, Saponins comprising of both aqueous and ethanolic extracts. In the comparative phytochemical analysis of the extract of Sida acuta and Dioscorea bulbifera, it was shown that ethanolic extraction had the highest numbers of phytochemicals extracted and the highest mean concentrations of the phytochemicals than the aqueous extraction. The quantitative comparism of the two forms of the extracts proved the ethanolic extracts to be better than the aqueous extracts based on the concentration and number of phytochemicals extracted, judging from the results of the p-values< 0.05 obtained, which justified the choice of the ethanolic extracts over aqueous extracts of Sida acuta and Dioscorea bulbifera in the research, except for Alkaloid that had p-values (0.419 and 0.184 respectively), which were not significantly different. However, Citrus aurantifolia was not involved in ethanolic and aqueous extraction because of its original liquid state which does not support any further liquid- liquid extraction. On the basis of concentration, Alkanoid of Citrus aurantifolia showed the highest mean concentration of 84.0 across all phytochemical extracted, followed by Tannin (50.00) of Sida acuta then Phenol (18.40) of Dioscorea bulbifera. However, the mean concentrations of the phytochemicals influenced the antimicrobial performances of the plant extracts in the hierarchy of Citrus aurantifolia, Sida acuta and Dioscorea bulbifera.
Identification of the test organisms was based on the most reliable identification technique known as the Polymerase Chain Reaction (PCR) and sequencing of the different organisms. In the agarose gel electrophoresis of the selected bacterial isolates, Lanes 1 – 3 represents 16SrRNA gene bands (1500bp) of Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae respectively. Lane L represents the 100bp Molecular ladder indicated at 500bp. The obtained 16SrRNA sequence from the isolate produced an exact match during the megablast search for highly similar sequences from the National Center for Biotechnology Information (NCBI) non-redundant nucleotide (nr/nt) data base,the 16SrRNA of the isolates (Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholerae) showed a percentage similarity to other species at 100%. The evolutionary distances computed using the Jukes-Cantor method were in agreement with the phylogenetic placement of the 16S rRNA of the isolates A1, A2 and M1 within the Escherichia, Salmonella and Vibriosp revealed a closely relatedness to Escherichia coli0157:H7, Salmonella typhimurium and Vibrio cholera respectively.
Antimicrobial susceptibility test of the plant extracts on the test organisms showed that the three plant extracts had varied degree of sensitivity and resistance. The organisms were most sensitive to Citrus aurantifolia, followed by Sida acuta and Dioscorea bulbifera. In contrast, the organisms showed the highest level of resistance to Dioscorea bulbifera followed by Sida acuta and least to Citrus aurantifolia. However, the overall antimicrobial susceptibility test of the plant extracts on test organisms was supported by the mean zone of inhibitions of extracts, with Citrus aurantifolia having highest zone, followed by Sida acuta and Dioscorea bulbifera having lowest zone in the single extracts. The order in the antimicrobial performance of the plant extracts is evident in the concentrations of the phytochemicals extracted, hence, the higher the concentration of phytochemicals the higher the antimicrobial activity. However, Citrus aurantifolia had the highest phytochemical concentration in Alkaloid, followed by Sida acuta in Tannin and the least phytochemical concentration was Dioscorea bulbifera in Phenol (See Table .1, .2 and 3).
Percentage distributions of the antimicrobial pattern of extracts on the test organisms showed the response of each of the test organisms to the extracts. Among the extracts, Escherichia coli0157:H7 responded highest, moderate and least sensitive to Sida acuta, Citrus aurantifolia and Dioscorea bulbifera respectively. Salmonella typhimurium was highly sensitive to Citrus aurantifolia and least equal sensitive to Sida acuta and Dioscorea bulbifera respectively. Whereas, Vibrio cholerae showed high equal percentage sensitivity to Sida acuta and Citrus aurantifolia and least sensitive to Dioscorea bulbifera. However, Sida acuta was most active on Escherichia coli0157:H7 followed by Vibrio cholerae and Salmonella typhimurium. While Citrus aurantifolia was most active on Salmonella typhimurium with an equal strength on Vibrio cholerae and Escherichia coli0157:H7. This finding did not completely agreed with previous research (WHO, 2017) which had Citrus aurantifolia as the most effective on Vibrio cholerae, Salmonnella typhimurium and Escherichia coli0157:H7 respectively. Dioscorea bulbifera was least active on Escherichia coli0157:H7 and most active with an equal strength on Salmonella typhimurium and Vibrio cholerae.
Considering different percentage concentrations of plant extracts of 100%, 75%, 50% and 25% against the test organisms, the mean zone of inhibitions was in the order of increasing concentrations. Thus, findings implied that the higher the concentrations the higher their zones of inhibition as supported the findings of Munoz et al.
Conclusion
Nature nurtures every creature with green plants and also provided medicinal properties in these plants in form of phytochemicals in the treatment of diseases.
In recent years, particular attention is being given on a substitute for a safe natural bio remedy for the therapy of infectious diseases because of their less or no harmful effects and resistance in microbes against them. The results of this study revealed that the different plants extracts were found to be very effective against the test organisms. So, it may provide new leads in the advancement of new antimicrobial compound for the therapy of other diseases yet to be discovered.
References
- Saganuwan SA (2012) Principles of Pharmacological Calculations. Zaria: AhmaduBello University 1st ed: 23-58.
- Kumar S, Mahanti P, Singh NR, Rath SK, Jena PK, et al. (2018) Antioxidant activity, antibacterial potential and characterization of active fraction of Dioscorea pentaphylla
- tuber extract collected from Similipal Biosphere Reserve, Odisha, India. Brazilian Journal of Pharmaceutical Sciences 53: 17006.
- Ghosh S, Nitnavare R, Dewle A, Tomar GB, Chippalkatti R, et (2015) Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: anticancer and antioxidant activities. International Journal of Nanomedicine 10: 7477- 7490.
- Evbuomwan L, Chukwuka EP, Obazenu EI, Ilevbare L (2018) Antibacterial activity of Vernonia amygdalina leaf extracts against multidrug resistant bacterial isolates. Journal of Applied Science and Environmental Management 22: 17-21.
- Tcheghebe OT, Seukep AJ, Tatong FN (2017) Ethnomedicinal uses, phytochemical and pharmacological profiles, and toxicity of Sida acuta Burm. f.: A review article. The Pharmacological Innovation 6: 1-6.
- Wizor CH (2012) Analysis of the Developmental Trends of Single Family Housing Estates in Port Harcourt Metropolitan Fringe Areas. Ph.D Dissertation, Department of Geography and Environmental Management, University of Port Harcourt.
- Ofomata GEK (1979) Nigeria in Maps: Eastern States. Ethiope Publishers.
- Azwanida NN (2015) A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 4: 2167-0412.
- Jukes TH, Cantor CR (1969) Evolution of protein In Munro HN, editor, Mammalian Protein Metabolism, Academic Press, New York 21-132.
- Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic Molecular Biology and Evolution 4: 406-425.
- Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783-791.
- Diep B, Moulin J, Bastic-Schmid V, Putallaz T, Gimonet J, et al. (2019) Validation protocol for commercial sterility testing Food control 103: 1-8.
- National Committee of Clinical Laboratory Standard (1993) Performance standards for antimicrobial disc for susceptibility test. Approved standards: CCC document M2 – A5, Pennsylvia:
- https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/ reference/ReferencesPapers.aspx?ReferenceID=1824862.
- Clinical and Laboratory Standard Institute (CLSI) (2016) Performance standards for antimicrobial susceptibility CLSI M100-S26.
- Clinical and Laboratory Standard Institute (CLSI) (2019) Performance standards for antimicrobial susceptibility testing; 29th informational CLSI document M100-S29. Clinical and Laboratory Standards Institute, Wayne, PA.

