Author(s): Ajayi AO*, Ayemidotun O, Ajayi NO and Yakubu P
This study shows the bactericidal effect of Electromagnetic Field on fruit juice microbes. Short shelf-life period of fruit juice caused by spoilage organisms has limiting factor for its economy value. The Eighteen microorganisms isolated from both fresh and spoilt fruit samples (Pineapple and Apple), and identified during the study include, twelve (12) bacteria and Six (6) fungi, out of which only the bacterial isolates were exposed to electromagnetic field of 0mG, 500mG, and 5000mG for thirty minutes. The bacteria species were Leuconostoc mesentroides, Bacillus species, Lactobacillus brevis, Microbacterium species, Clostridium species, Bacillus cereus, Acetobacter aceti, and Staphylococcus aureus. The Gram negative bacteria isolates were Erwinia carotovora, Erwinia ananas, and Proteus species. Exposure of the isolates to an electromagnetic field of 0mG, 500mG and 5000mG showed a decrease in some electromagnetic field magnitude. This study shows reduction in growth range among most bacterial species tested at 500mG electromagnetic radiation exposure, but the growth of many of these bacterial species were triggered at 5000mG electromagnetic radiation exposure. This may mean an initiation of: adaptation mechanism, growth mechanism in some microorganism, and sugar content of the fruit juice from which they are being isolated. The exposure of the bacteria to electromagnetic field elicited detectable responses therefore depends on the adaptation mechanism of each bacteria and sugar content of the fruit from which it is being isolated from. Thus, future research can be done to optimize the limits specified for target microbes that are strength and frequency of this EMF in diseases control.
The effects of electromagnetic radiation (EMR) on living organisms including bacteria have significantly increased in recent years. This interest is caused first of all; the fact that EMR is widely applied in telecommunication technology and that its influence on bacteria can lead, in a varying degree to a change of their role in human and animal diseases [1]. In their study shows that the exposure of Escherichia coli and Pseudomonas aeruginosa to extremely low frequency electromagnetic field (2Mt;50Hz) at 4,6 and 8h of incubation [2]. The number of cell was significantly decreased in bacteria exposed to electromagnetic field when compared with the control [3]. The antimicrobial effect of oscillating magnetic field pulses is not due to the temperature effect, but rather to the ability to cause damage [5].
Early in the 20th century, Nobel laureate Hendrik Antoon Lorentz took the electrodynamics theory further to the microscopic scale and also laid the foundation for the special theory of relativity, formulated by Nobel laureate Albert Einstein in 1905. In the 1930s Paula. M. Dirac expanded electrodynamics to a more symmetric form, including magnetic as well as electric charges. With his relativistic quantum mechanics, he also paved the way for the development of quantum electrodynamics (QED) for which Richard P. Feynman, Julian Schwinger, and Sin-Itiro Tomonaga in 1965 received their Nobel prizes [6]. The electric field of a point charge can be obtained from Coulomb’s law:
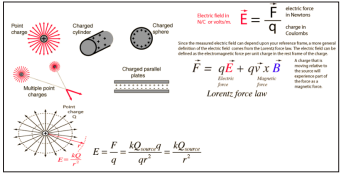
Figure 1: Source: (Nave 2012)
The electric field is radially outward from the point charge in all directions. The circles represent spherical equipotential surfaces. The electric field from any number of point charges can be obtained from a vector sum of the individual fields. A positive number is taken to be an outward field; the field of a negative charge is toward it [6].
Magnetic fields are produced by electric currents, which can be macroscopic currents in wires, or microscopic currents associated with electrons in atomic orbits. The magnetic field B is defined in terms of force on moving charge in the Lorentz force law. The interaction of magnetic field with charge leads to many practical applications. Magnetic field sources are essentially dipolar in nature, having a north and south magnetic pole. The SI unit for magnetic field is the Tesla, which can be seen from the magnetic part of the Lorentz force law F magnetic = qvB to be composed of (Newton x second)/(Coulomb x meter). A smaller magnetic field unit is the Gauss (1 Tesla = 10,000 Gauss) [6]. Electromagnetic radiation (EM) is described in terms of its wavelength, frequency, or energy.
The relationship between energy and frequency is given by the equation, E = hv, where h is Planck’s constant. A direct relationship exists; electromagnetic radiation with a higher frequency is more energetic.

Plate 1: The Pineapple. (Source: PBPM, 2009)
The pineapple belongs to the bromeliad family, which contains 50 genera and about 2,500 known species, all but one of them from Central and South America. The exact origin of the cultivated species - Ananas comosus var. comosus- is hard to pinpoint, but Ananas comosus var. ananas soides (with very small, seedy fruit and spiny leaves) is considered a wild ancestor of the domestic pineapple. Its origins are in Brazil/Paraguay straddling the equator between latitudes 15°N &30°S (In Australia they are commercially grown between 15° & 27°S, Cooktown to Brisbane). Domestication is thought to have occurred in the Guianas [7].
Nutritional value per 100g(3.5oz) of pineapple are; Energy :209KJ (50kCal) Carbohydrate:13.12g Sugars:9.85g Dietary fiber:1.4g
Fat:0.12g Protein:0.54g Vitamin(Thiamin(B1):0.079mg, Riboflavin(B2):0.032mg, Nacin(B3):0.5mg Pantothenic acid:0.213 Vitamin B6:0.112mg Folate(B9):18mg Choline:5.5mg Vitamin C:47.8mg), and its mineral content includes:Calcium:13mg, Iron:0.29mg,, Magnesium:12mg, Manganese:0.927mg, Phosphorus:8mg, Potassium:109mg, Sodium:1mg, Zinc:0.12mg (National Nutrient Database, 2015).

Plate 2: Green and Red Apple (Source: Getty image, 2014).
The apple is the most ubiquitous of temperate fruits and has been cultivated in Europe and Asia from antiquity. It was known to the Greeks and Romans and mentioned by Theophrastus in the third century B.C. Since then the apple has been distributed into almost all parts of the world. The genetic variability found in the apple has allowed adapted types to be selected for different environments, and selection continues for new types to extend apple culture into both colder and warmer regions. Orchards are now found in Siberia and northern China where winter temperatures fall to -40°C and in high elevations in Colombia and Indonesia straddling the equator where two crops can be produced in a single year [8]. Present world production of apples (FAO 1995) is close to 49 million tonnes. Apples are the fourth fruit crop in importance after all citrus (85 million tonnes), grapes (56 million tonnes), and banana (53 million tonnes).
Electromagnetic fields may have different targets affecting the biological systems. At cell level one of the possible ways to evidentiate the biological effects of electromagnetic fields are probably mediated by plasma membrane [9]. Lethality of irradiation depends on the target (microorganism), condition of the treated item, and environmental factors. Addition or removal of salt or water, time/temperature, or oxygen presence is factors that will influence the antimicrobial effect of irradiation. Irradiation is considered an additive in the U.S. and as such, it needs to be approved by the FDA office of premarket approval for each new application and labeled [10].
Use of radio waves to make safer fruit juices has been worked out by researchers for many years but has not been commercialized yet due to economic reasons. Conventional pasteurization is done using different heating techniques, but they can affect the nutrient composition and flavour of the fruit and vegetable juices. The new method is totally different. The radio frequency electric fields inactivate bacteria in apple juices without heating them. According to, the method has been used half century ago for pasteurization purposes but this is the first time that they could inactivate bacteria of fruit juice using this technique successfully. However, using the combined method, namely the use of moderate heat in addition to the none-thermal method, has much greater effects than those of the either processes has alone. They have built a specially designed treatment to apply high-intensity radio frequency electric fields to apple juice [11].
Levels of electromagnetic fields (EMF) from human-made sources have increase steadily over the past 50-100 years. Most EMF exposures come from increased use of electricity and new technologies. In the past decades, potential adverse effects from EMF exposure on human health have been an important topic of research. The limited numbers of published studies addressing the risk of EMF to terrestrial and aquatic ecosystems show little or no evidence of a significant environmental impact, except for some effects near very strong sources. From current in formation the exposure limits in the ICNIRP guidelines for protection of human health are also protective of the environment [12].
In this study, the relative effects of electromagnetic radiation (EMR) on the isolated microbes from some fruit samples specifically Pineapple (Cosmos ananas) and Apple (Malus domestica) were determined with the aim of monitoring and controlling the rate of fruit spoilage and fruit juice storage by using electromagnetic field (EMF).
Two species of Malus domestica(Red apple and Green apple) and Cosmos ananas (Sugar loaf pineapple) that is of both small and big size comprising both fresh and spoilt samples were bought from three locations which are Akungba Akoko,Akure, and IkareAkoko, Ondo State, Nigeria.
The laboratory was cleaned and laboratory bench was swabbed. All the glassware used for the study were adequately washed and sterilized in the oven at 160ºC for One hour. Liquid media used were sterilized in the autoclave at 121ºC for 15minutes, and then cooled to 45ºC before pouring into plates. Serial dilutions of the samples were made, 1mL of appropriate diluents incubated at 37ºC for 18 to 24 hours and 25ºC for 3 to 5days for bacteria and fungi respectively. Specimens showing no growth or lower colony counts were incubated for another period (24 hrs) of time. At the end of which the final colony forming units (CFU) and spore counts were taken. All organism isolated were Microscopically and biochemically identified using standard microbiological measures. The identification of of fungi were based on their cultural and morphological characteristics determined by lactophenol cotton blue stain technique as well as related biochemical characteristics confirmed with the use of the compendium of fungi.
Isolates from the stock were transferred unto freshly prepared plates and afterwards cultured in liquid nutrient medium (broth). From every breed, 1ml inoculums were introduced into 9ml freshly prepared broth in test tubes. The test tubes were treated with magnetic field of 500 and 5000mG for 15 minutes by placing the test-tubes in front of the coil of wires producing electromagnetic field at different position. The untreated breeds were taken as control.
After the treatment with magnetic field, the isolates were seeded on Petri dish containing selected medium, incubated at 37°C for 24 hours and 25°C for 3-5 days. The same process was done for the untreated ones. The colony count was done for the bacteria to determine the effect of magnetic field.
Eighteen (18) microorganisms were isolated from both fresh and spoilt fruit samples (Pineapple and Apple), out of which twelve (12) were bacterial isolates and six (6) were fungi isolates of which only the bacterial isolates were expoused to electromagnetic field of 0mG, 500mG, and 5000mG for thirty (30) minutes. The bacteria isolates were coded as PA1, PA2, RA1, RA2, RA3, GA1, GA2, GA3, sPa1, sPa2, sGA and sRA. They were identified to be Leuconostoc messentroides, Bacillus species, Clostridium species, Bacillus cereus, Acetobacter aceti, Lactobacillus species, Lactobacillus brevis, Mycobacterium spp, Erwinia carotovora, Erwinia ananas, Proteus species and Staphylococcus aureus (Table1). While the fungi isolates were coded as FPA1, FPA2, FGA1, FGA2, FGA3, FRA and were identified to be Aspergillus niger, Aspergillus aculeatus, Rhizopus spp, Aspergillus carbonarius, Aspergillus aculeatus, and saccharomyces cerevisae (Table 2) Table 3 shows the microbial growth count (in CFUs) after exposure to EMF. There were reduction in growth range among most bacterial species tested at 500mG electromagnetic radiation exposure, but the growth of many bacterial species were triggered at 5000mG electromagnetic radiation exposure isolates. The bacterial growth values in broth cultures of are also shown in Tables 4 and 5. Figures 2 to 6 shows the effect of electromagnetic field on various microbial species identified and treated for 30 minutes of varied frequency with their corresponding responses of reduction of microbial load for Leuconostoc messentroides and Staphylococcus aureus. Nevertheless, there were variations for others in most instances. This can be based on some physiological conditions.
Table1: Characteristics of bacterial isolates
| Isolates | Shape | Gram’sreaction | Arabinose | Galactose | Rafinose | Sucrose | Maltose | Lactose | Oxidase | Catalase | Probable organism |
| PA1 | Sphere | + | A | A | A | A | A | A | - | + | Leuconostoc messentroides |
| PA2 | Rods | + | A | A | A | A | A | A | + | + | Bacillus species |
| RA1 | Curved rod | + | - | AG | AG | AG | AG | AG | - | - | Lactobacillus species |
| RA2 | Curvedrod | + | A | A | - | A | A | - | - | Lactobacillus brevis | |
| RA3 | Rod | + | A | A | - | A | A | A | + | Mycobacteriumspecies | |
| GA1 | Rod | + | AG | AG | AG | AG | AG | AG | - | - | Clostridium species |
| GA2 | Rod | + | - | A | A | A | A | - | + | + | Bacillus cereus |
| GA3 | Rod | + | - | - | - | - | - | - | - | + | Acetobacter aceti |
| sPa1 | Rod | - | - | A | A | A | A | A | - | + | Erwinia ananas |
| sPa2 | Spherical | + | A | A | - | A | A | A | - | + | Staphylococcus aureus |
| sGA | Rod | - | - | - | - | A | - | - | - | Proteus species | |
| sRA | Rod | - | AG | AG | AG | AG | AG | - | - | + | Erwinia carotova |
Keys: PA1 and PA2: Isolates from big and small fresh Pineapple sample respectively. RA1, RA2 and RA3: Isolates from fresh Red Apple. GA1, GA2, and GA3: Isolates from fresh GreenApple. sPA1: Isolates from big spoilt Pineapple sPA2: Isolates from small spoilt Pineapple sGA: Isolates from spoilt Green Apple sRA: Isolates from fresh Red Apple (+):Positive (-):Negative (A/G):Acid/Gas production
| Isolates | Colour | Texture of Conidia | Shape and Ornamentation of Conidia | Probable Fungi |
| FPA1 | Dark brown | Finely wrinkled | Ellipsoidal and Warty | Aspergillus niger |
| FPA2 | Grey tone | Spiny | Ellipsoidal and Echinulated | Aspergillus aculeatus |
| FGA1 | White to Grey colour | Rough | Collumella rhizoids | Rhizopus species |
| FGA2 | Black | Wrinkled | Globular and Warty | Aspergillus carbonarius |
| FGA3 | Grey tone | Spiny | Ellipsoidal and Echinulated | Aspergillus aculeatus |
| FRA | White to Cream colour | Smooth | Globular and Ellipsoidal | Saccharomyces cerevisae |
KEYS:
FPA: Isolate from Pineapple
FGA: Isolate from Green Apple
FRA: Isolate
Table 3: Microbial growth count (in CFUs) after exposure to EMF
| Isolates | Control0mG(Control) | 500mG | 5000mG |
| PA1 | 196 | 184 | 201 |
| PA2 | 46 | 30 | 56 |
| RA1 | 12 | 7 | 19 |
| RA2 | 60 | 19 | 600 |
| RA3 | 194 | 12 | 120 |
| GA1 | 45 | 34 | 62 |
| GA2 | 50 | 48 | 53 |
| GA3 | 57 | 38 | 570 |
| sPa1 | 201 | 33 | 26 |
| sPa2 | 263 | 86 | 52 |
| sGA | 96 | 30 | 20 |
| sRA | 132 | 29 | 17 |
Table 4: Bacterial growth values in broth culture
| Isolates | Control 0mG(Control) | 500mG | 5000mG |
| Isolates | 0mG | 500mG | 5000Mg |
| PA1 | 1 | 0.938 | 1.026 |
| PA2 | 1 | 0.652 | 1.271 |
| RA1 | 1 | 0.583 | 1.583 |
| RA2 | 1 | 0.317 | 10 |
| RA3 | 1 | 0.062 | 0.619 |
| GA1 | 1 | 0.756 | 1.378 |
| GA2 | 1 | 0.960 | 1.06 |
| GA3 | 1 | 0.667 | 10 |
| Spa1 | 1 | 0.164 | 0.129 |
| Spa2 | 1 | 0.327 | 0.198 |
| sGA | 1 | 0.312 | 0.208 |
| sRA | 1 | 0.300 | 0.129 |
Table 5: Percentage of bacterial growth values in broth cultures
| Isolates | Control 0mG(Control) | 500mG | 5000mG |
| PA1 | 100 | 93.90 | 102.6 |
| PA2 | 100 | 65.20 | 121.7 |
| RA1 | 100 | 58.30 | 158.3 |
| RA2 | 100 | 31.70 | 1000 |
| RA3 | 100 | 6.20 | 61.9 |
| GA1 | 100 | 96.00 | 106 |
| GA2 | 100 | 66.70 | 10 |
| GA3 | 100 | 75.60 | 137.8 |
| sPAI | 100 | 16.40 | 12.9 |
| sPA2 | 100 | 32.70 | 19.8 |
| sRA | 100 | 31.20 | 20.8 |
| sGA | 100 | 30.00 | 12.9 |

Figure 2: Effect of electromagnetic field on PA1 - Leuconostoc messentroides and PA2 - Bacillus species treated for 30 minutes at varied frequency
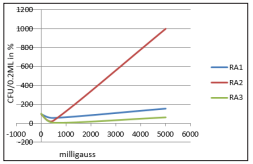
Figure 3: Effect of electromagnetic field on RA1 - Lactobacillus species, RA2 - Lactobacillus brevis and RA3 - Microbacterium species treated for 30 minutes at varied frequency
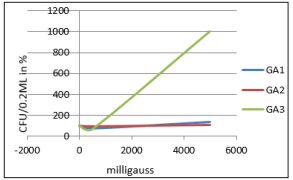
Figure 4: Effect of electromagnetic field on GA1 - Clostridium species, GA2 - Bacillus cereus and GA3 - Acetobacter aceti treated for 30 minutes at varied frequency
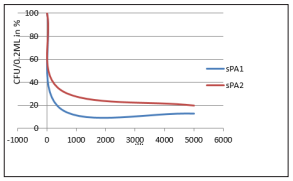
Figure 5: Effect of electromagnetic field on PA1 - Leuconostoc messentroides and sPA2 - Staphylococcus aureus treated for 30 minutes at varied frequency
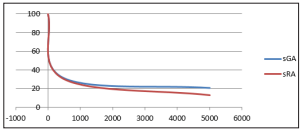
Figure 6: Effect of electromagnetic field on sGA - Proteus species and sRA - Erwinia carotova treated for 30 minutes at varied frequency
The bactericidal effect of Electromagnetic Field (EMF) on fruit juice microbes using apple and pineapple as a case study expressed in a significant decrease in some microorganism and increase in some as shown by this study. This study also shows the protective or reparation mechanisms and initiation of some growth abilities in some microorganism as well as the potential of EMFs to kill some essential particles in some microorganisms. It can be seen that the electromagnetic field causes a decrease of growth in CFU in all samples at certain level of exposure. This correlates with the study of Ajayi and Ajayi [4].
The result from the exposure of the isolates to an electromagnetic field of 0mG, 500mG and 5000mG showed a decrease in some electromagnetic field magnitude. This corroborates with the study of which shows relative variation of electromagnetic field on viable cells [1].
This study showed a decrease in the growth rate of exposed samples at 500mG and increase in the growth rate of exposed samples at 5000mG with respect to control. This finding correlates with the report of [13]. sPA1, spa2, sGA and sRA recorded a regular decrease in colony forming unit as field increases from 500mG to 5000mG. This work is in agreement with who found that exposure of apple juice to high-intensity radio frequency electric fields inactivate bacteria. The exposure of the bacteria to electromagnetic field elicited detectable responses depending on the adaptation mechanism of each bacteria and sugar content of the fruit from which it is being isolated from. Similarly, may have a beneficial or harmful effect which depends on limit that are strength and frequency [11-18].
