Author(s): Ahmed Qasem*, Ranim Karroum , Mohammed Khadoor and Taym Darwish
Neurotrophic keratitis is a degenerative corneal disease caused by impairment of corneal sensory innervation. It is characterized by decreased or absent corneal sensation, leading to epithelial breakdown, impairment of healing, and ultimately the development of corneal ulceration, melting, and perforation [1]. Neurotrophic keratopathy (NK) is a rare, that affects <1.6 per 10,000 people [2,3].
Every ocular or systemic condition altering corneal sensory innervation, which runs from the cornea itself to the pontine trigeminal nucleus—can result in NK. The most common ocular conditions associated with NK are herpes keratitis (zoster and simplex), topical anesthetic abuse, chemical and physical burns, contact lens abuse, topical drug toxicity, irradiation to eye or adnexa, and corneal surgery [4].
The most common presenting symptoms of NK include redness, sensitivity to light, dry eye, reduced visual acuity, blurred vision, and eye fatigue [5]. The most frequently reported patient complaints to include driving impairment, reading impairment, difficulty watching television, and concern with potentially losing their eyesight due to NK [5].
The Mackie classification scheme identifies neurotrophic keratopathy fitting into one of three stages [2,6]. Stage 1 is characterized by epithelial alterations, such as punctate keratopathy, hyperplasia, irregularity, and superficial neovascularization. Rose Bengal staining of the conjunctiva can show epithelial compromise. Stage 2 has persistent epithelial defects (PED) that are commonly found in the superior half of the cornea. Surrounding epithelial edema can easily detach and enlarge the defect. The edges of the PED can appear smooth with rolled edges. Stage 2 can also present with folds in Descemet’s membrane and an inflammatory reaction in the anterior chamber. Stage 3 is characterized by corneal ulcers that may progress to perforations or stromal melting.
Early diagnosis, treatment and careful monitoring of neurotrophic keratitis patients are mandatory to achieve epithelial healing and prevent progression of corneal damage. The use of preservative- free artificial tears may help improve the corneal surface at all stages of disease severity.
In the event of stromal melting, use of topical collagenase inhibitors, such as N-acetylcysteine, and systemic administration of tetracycline or medroxyprogesterone may be considered. Use of topical antibiotic eye drops to prevent infection in eyes with neurotrophic keratitis at stages 2 and 3 are recommended. Topical nerve growth factor (NGF) and autologous serum eye drops are considered as promising treatments of neurotrophic keratopathy [7].
Surgical treatments are reserved for refractory cases. They include partial or total tarsorrhaphy, amniotic membrane transplantation, conjunctival flap, and Botulinum A toxin injection of the eyelid elevator muscle.
Neurotization surgery with direct transfer of the supratrochlear or supraorbital nerves to the subconjunctival space has shown promise [8-11]. Early diagnosis, severity-based treatment, and careful monitoring of NK patients are mandatory to achieve epithelial healing and prevent progression of corneal damage, especially since worsening of NK is frequently asymptomatic.
All topical medications should be discontinued because they have detrimental effects on the ocular surface epithelium. Additionally, all ocular surface-associated diseases, such as exposure keratitis, dry eye, and limbal stem cell deficiency, which may worsen the prognosis of NK, need to be taken care of. For instance, it will be necessary to correct any eyelid dysfunction, to consider punctal occlusion, and/or perform limbal cell transplantation [2-12].
Treatment of NK should be based on disease severity. Treatment for stage 1 disease aims at improving epithelial quality and transparency and avoiding epithelial breakdown. In the presence of PED, therapy is aimed at preventing stromal involvement and corneal ulcer formation as well as promoting corneal healing. More severe cases, with corneal ulcer and stromal melting, require immediate attention to stop the stromal lysis and prevent perforation [2,12,13]. Topical steroids have been proposed for NK to control ocular inflammation (if present); however, steroids may increase the risk of corneal melting and perforation by inhibiting stromal healing, and their use should be considered with great caution [2,12,14]. Topical nonsteroidal anti-inflammatory drugs may also inhibit the healing process and should be avoided [15]. In the event of stromal melting, use of topical collagenase inhibitors, such as N-acetylcysteine, and systemic administration of tetracycline or medroxyprogesterone may be considered [16,17]. Use of topical antibiotic eye drops to prevent infection in eyes with NK at stages 2 and 3 is recommended.
Nonpharmacological treatments for NK include therapeutic corneal or scleral contact lenses in the event of PED to promote corneal epithelial healing [16]. Contact lens use may increase the risk of secondary infections [18].
New surgical and medical alternatives have recently been introduced. The surgical treatments are transplantation of amniotic membranes (AMs), conjunctival flaps and sympathectomy, but the most recent research considers that medical therapy can restore the nerve damage that forms the basis of the pathology. These new medical treatments include autologous serum eye drops, umbilical cord serum eye drops and neurotrophin eye drops [19].
The mechanism of insulin in promoting cornea wound healing in our patients remains speculative, but data suggest that restoration of corneal nerves and/or improved epithelial cell migration may play key roles. In diabetic mice, topical insulin has been shown to slow the loss of sub-basal plexus corneal nerves [20]. Furthermore, the addition of insulin promoted cell migration and closure of artificial wounds in cultured sheets of corneal epithelial cells in an in vitro model of corneal epithelial wound healing [21].
Insulin-like growth factor-1 (IGF-1) has been shown to be an important modulator of corneal wound healing. In several pre- clinical studies, IGF-1 was shown to act synergistically with substance-P to promote corneal epithelium wound healing [22]. In two case series, patients with neurotrophic corneal epithelial defects treated with a topical combination of substance P-derived peptides and either IGF-1 or IGF-1-derived peptides underwent complete epithelial resurfacing within 4 weeks, at response rates of 89% and 73% [23, 24]. Topical insulin may be a simple and effective treatment for refractory neurotrophic corneal ulcers [25].
The prognosis of NK depends on several factors, namely, the cause of impairment of corneal sensitivity, the degree of corneal hypoesthesia and the presence of concomitant diseases of the ocular surface. Obviously, the more severe the corneal sensory impairment, the greater the likelihood of disease progression [26].
A 62-year-old male patient of Arabian descent was referred to our hospital for the management of stage III NK in the left eye. History started 18 months ago; when he underwent uneventful phacoemulsification cataract surgery elsewhere. The post- operative period was unremarkable. Six months ago, the patient developed a sudden onset of left eye diminution of vision, red eye, and tearing. The patient sought ophthalmic consultation and was diagnosed with herpes simplex keratitis. The patient is covered with local and systemic antiviral, antibiotics, and lubrication. The patient was followed multiple times by his ophthalmologist for 4 months; no improvement was achieved.
Six weeks ago, the patient was referred to us with stage III NK in the left eye with persistent non-healing epithelial defect. On examination, visual acuity OS was hand motion (HM), severe conjunctival injection, deep corneal vascularization, corneal opacity with stromal infiltration, epithelial defect about 3.5 x 6 mm as shown in (Fig. 1), stromal thinning, Descemet membrane folds and Loss of corneal sensation by cotton wisp test. No purulent discharge, negative Seidel test, no AC reactivity, no hypopion, normal iris, and pupil was round, regular, and reactive. IOP within normal range. The swab was taken for culture and sensitivity, which revealed no growth for bacteria and fungi.
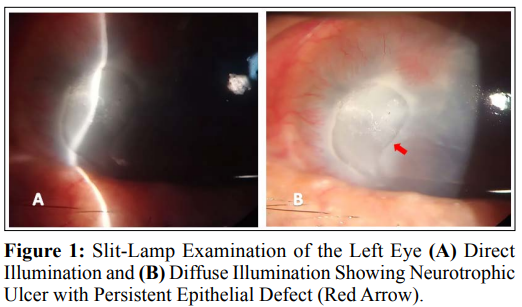
The patient is treated with insulin eye drops mixed with artificial tears (glycerin 0.2%, hypromellose 0.2%, polyethylene glycol 400:1%). Insulin (soluble insulin 30% and isophane insulin 70%) 2ml mixed with 8 ml of artificial tears (glycerin 0.2%, hypromellose 0.2%, polyethylene glycol 400:1%). Used 4–5 times daily and kept in refrigerator 5°c. The drops are renewed every 7 days. Preservative free levofloxacin 1.5% eye drops are prescribed for patient as a prophylaxis against secondary bacterial infection. The Patient followed weekly for 6 weeks to monitor case progression and report any side effects; After 4 weeks, the epithelial defect showed substantial healing that cover the epithelial defect completely at 6 weeks and was confirmed by negative fluorescein staining as shown in (Fig. 2). No side effects of insulin eye drops were reported.
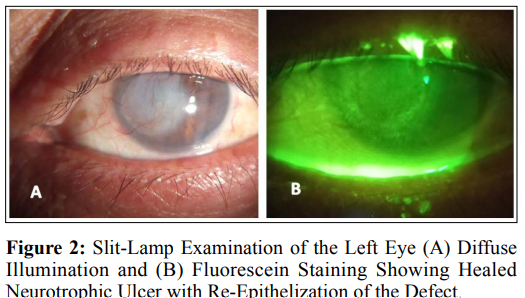
Insulin drops have been shown to substantial promotion of persistent epithelial defect healing in the case of neurotrophic ulcers. Therefore, topical insulin use can potentially help in the treatment refractory neurotrophic keratitis. Further study with large case numbers and long follow-up should be the main focus of future research.
Neurotrophic Keratitis; PED: Persistent epithelial defect; NGF: Nerve growth factor; IGF-1: Insulin-like growth factor-1; AMs: Amniotic membranes; IOP: Intraocular pressure; AC: Anterior Chamber
Written informed consent was obtained from the patient for participation publication of this paper and any accompanying images.
The authors declare that there is no conflict of interest regarding the publication of this paper. This study received no specific grant from any funding agency in the public, commercial, or not-for- profit sectors.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
AQ described the ocular changes, prepare the drops and wrote the manuscript. RK and MK both contributing in writing manuscript. TD supervision and review. All authors read and approved the final manuscript.
The authors do not have any acknowledgments to state. No financial support was received.
1.Semeraro F, Forbice E, Romano V, Angi M, Romano MR, et al. (2014) Neurotrophic keratitis. Ophthalmologica 231: 191-197.
2.Bonini S, Rama P, Olzi D (2003) Neurotrophic keratitis. Eye 17: 989-995.
3.Sacchetti M, Lambiase A (2014) Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol 8: 571-579.
4.Vatinee Y Bunya, Maria A Woodward, Alessandro Rabiolo, Giulio Ferrari, Paolo Rama, et al. (2023) Neurotrophic Keratitis -EyeWiki. (n.d.). Eyewiki.aao.org. Retrieved 1-5.
5.Murray LT, McCormack J, Grobeiu I (2020) Development of the neurotrophic keratopathy questionnaire: qualitative research. J Patient Rep Outcomes 4: 30.
6.Mackie IN, Keratitis I, Fraunfelder F (1995) editors. Current ocular therapy. Philadelphia, PA: WB Saunders 452-454.
7.Lee YC, Kim SY (2015) Treatment of neurotrophic keratopathy with nicergoline. Cornea 34: 303-307.
8.Pérez-Bartolomé F, Mingo Botín D, de Dompablo E, de Arriba P, Arnalich Montiel F, et al. (2019) Post-herpes neurotrophic keratopathy: Aetiopathogenesis, clinical signs, and current therapies. Arch Soc Esp Oftalmol (Engl Ed) 94: 171-183.
9.Voelker R (2018) New Drug Treats Rare, Debilitating Neurotrophic Keratitis. JAMA 320: 1309.
10.Scanzera AC, Shorter E (2018) Case Series: Management of Neurotrophic Keratitis from Familial Dysautonomia. Optom Vis Sci 95: 678-681.
11.Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC (2018) Neurotrophic keratitis: current challenges and future prospects. Eye Brain 10: 37-45.
12.Lambiase A, Rama P, Aloe L, Bonini S (1999) Management of neurotrophic keratopathy. Curr Opin Ophthalmol 10: 270-276.
13.Nishida T, Yanai R (2009) Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol 20: 276-281.
14.Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y (1982) Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol 66: 705-708.
15.Hersh PS, Rice BA, Baer JC (1990) Topical nonsteroidal agents and corneal wound healing. Arch Ophthalmol 108: 577-583.
16.Grey F, Carley F, Biswas S, Tromans C (2012) Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye 35: 288-291.
17.Portnoy SL, Insler MS, Kaufman HE (1989) Surgical management of corneal ulceration and perforation. Surv Ophthalmol 34: 47-58.
18.Khokhar S, Natung T, Sony P (2005) Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea 24: 654-660.
19.Lee SH, Tseng SC (1997) Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 123: 303-312.
20.Chen DK, Frizzi KE, Guernsey LS, (2013) Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst 18: 306-315.
21.Shanley LJ, McCaig CD, Forrester JV (2004) Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci 45: 1088-1094.
22.Nishida T, Yanai R (2009) Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol 20: 276-281.
23.Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, et al. (2007) Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol 51: 442-447.
24.Yamada N, Matsuda R, Morishige N, Yanai R, Chikama T, et al. (2008) Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 92: 896-900.
25.Wang A L, Weinlander E, Metcalf B M, Barney N P, Gamm D M, et al. (2017) Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 36: 1426-1428.
26.Cobo LM (1988) Corneal complications of herpes zoster ophthalmicus. Prevention and treatment. Cornea 7: 50-56.
View PDF