Author(s): Jeevan S Ladi*, Krutika J Ladi, Nitant A Shah and Ujwala M Gaikwad
Purpose: The lenticular diameter was reduced to below standard 6.5 mm, in a stepwise manner up to 5.2 mm and report on the Residual Stromal Thickness (RST), refractive outcomes and subjective scotopic visual quality of patients compared to those who underwent Small Incision Lenticule Extraction (SMILE) with the conventional 6.5 mm lenticular diameter.
Study Design: Retrospective study Methods: Patients undergoing SMILE in the first year of the study were subjected to lenticular diameter of standard 6.5 mm for lenticule extraction. In the second year, using the same nomogram, the lenticular diameter was gradually reduced from 6.4 to 6 mm, with 0.1 mm decrements in each subset. In the last 6 months, all cases were performed using the same nomogram but with lenticular diameter below 6 mm, again with 0.1 mm decrements in each subset.
Results: We included 100 eyes of 55 patients with a mean age of 26.18+ 6.6 years (median=25 years, IQR= 22 to 28 years). The mean preoperative best corrected visual acuity (BCVA) was 0.03+ 0.07 logMAR (range = 0 to 0.3 logMAR) and the mean preoperative spherical equivalent was -5.5D + 2.05D (median = -5.5D, IQR= -7.10 to -3.75D). The mean keratometry was 44.12D + 1.3D (range = 41.5 to 48D) and mean central corneal thickness was 532.24+ 29.8 µ (median = 533 µ IQR= 511 to 552 µ, range = 449 to 629 µ). The pupillary size in the study group was 3.80+ 0.82 mm (median= 3.84 mm, IQR= 3.3 to 4.3mm). At 1 month follow up; the best corrected distance visual acuity (UCDVA) across groups was 0.02 + 0.06 logMAR (median = 0 logMAR, range = 0 – 0.3 logMAR). There were no differences in UCDVA and contrast sensitivity between groups with respect to the lenticular diameter. Univariate linear regression analysis showed that age, spherical equivalent, preoperative pachymetry and lenticular diameter size were significantly associated with residual stromal thickness.
Conclusion: We found that SMILE performed with a lenticular diameter of < 6 mm was effective in delivering excellent refractive correction and improving the corneal biomechanical strength by virtue of significantly greater RST
Ever since the introduction of laser in situ keratomileusis (LASIK) procedure in 1990 by Pallikaris et al, there have been continuous refinements in refractive surgery, including wavefront guided LASIK and femtosecond-LASIK, to make treatments safer, more efficacious and improve patient satisfaction [1-3]. The main drawback of LASIK was the risk of corneal ectasia with a reported incidence of 0.06 to 0.8% and depends upon the degree of refractive error, preoperative pachymetry, thickness of the residual stromal bed and the preoperative corneal topography findings[4-6]. Ectasia was attributed to the creation of the corneal flap that reduced the biomechanical strength of the cornea [7].
To overcome this, and theoretically reduce the risk of ectasia, refractive lenticule extraction (ReLEx) was introduced about a decade ago [8]. This was a flapless surgery where the femtosecond laser was used to create an intrastromal lenticule that was extracted either through a larger ring shaped incision (FLEx) or through a 3mm small incision (SMILE) [9,10]. The ReLEx-SMILE procedure has been shown to be effective in treating myopia upto -10 D sphere and -5 D cylinder with no flap related complications like LASIK. It also has a lower incidence of dry eye, diffuse lamellar keratitis and epithelial ingrowth making it, perhaps, the safest corneal refractive procedure available today [9]. Yet, corneal ectasia following ReLEx –SMILE has been reported in the past, mostly in eyes with subclinical keratoconus [11,12]. Though one would expect SMILE to be potentially safer than LASIK in eyes with borderline topography due to the lack of a corneal flap, the residual stromal thickness (RST) may be the most important determinant predicting ectasia, irrespective of the type of procedure [7].
Currently, VisuMax (Carl Zeiss Meditec AG, Jena, Germany) femtosecond laser platform is the only one available for performing the SMILE procedure and the manufacturer recommends using a 6.5mm lenticule diameter for optimum results. We hypothesized that reducing this lenticular diameter may theoretically lead to reduction of the lenticule thickness extracted, thereby increasing the RST. In this study, we reduced the lenticular diameter below 6.5mm, in a stepwise manner up to 5.2 mm, and report on the RST, refractive outcomes and subjective scotopic visual quality of patients compared to those who underwent SMILE with the conventional 6.5mm lenticular diameter.
This was a retrospective study conducted at a tertiary refractive surgical practice in western India. The study was approved by the institutional ethics committee and followed the tenets of the declaration of Helsinki. All surgical procedures were performed after obtained expressed written consent from patients.
All patients who underwent SMILE between April 2016 and June 2018 with at least 1-month follow up were included in the analysis. Preoperative work up included best corrected distance visual acuity (BCDVA), detailed slit lamp and dilated fundus evaluation, refractive error measurement using objective Autorefractometer (Topcon, Tokyo, Japan) and subjective methods to obtain the best spherical and cylindrical correction and contrast sensitivity assessment using the Pelli-Robson’s chart at 1 meter distance. The central corneal thickness, kerometry values for the steep and flat corneal meridians and scotopic pupil size were measured using placido disk topography with Sheimpflug tomography based system (Sirius, CSO, Italy).
Patients underwent the SMILE surgery using standard surgical techniques described previously using the VisuMax (Carl Zeiss Meditec AG, Jena, Germany) laser platform [9]. The spherical and cylindrical correction values were used in the nomogram provided by the manufacturer before performing SMILE. Patients undergoing SMILE in the first year of the study were subjected to lenticular diameter of 6.5mm for lenticule extraction. In the second year, using the same nomogram, the lenticular diameter was gradually reduced from 6.4 to 6 mm, with 0.1 mm decrements in each subset, the number of patients in each subset was randomly chosen and was at the discretion of the operating surgeon. In the last 6 months, all cases were performed using the same nomogram but with lenticular diameter below 6 mm, again with 0.1 mm decrements in each subset.
Postoperatively, patients were prescribed topical antibiotics and topical steroids for 1 week in a tapering fashion; and lubricating eye drops for 1 month. Patients underwent detailed slit lamp evaluation and determination of uncorrected distance visual acuity (UCDVA) and contrast sensitivity at postoperative day 1 and at 1 month follow up. At each visit, patients were also shown standard photographs to demonstrate glare and halos and were asked whether they experienced any glare/haloes in each eye individually during the postoperative period. The residual stromal thickness was obtained automatically from VisuMax laser platform.
All continuous variables were presented as mean with standard deviation or median with interquartile (IQR) range and categorical variables were presented as proportions (n, %). Snellen’s visual acuity measurements were converted to logarithm of minimal angle of resolution (logMAR) and spherical equivalent was calculated as sphere + ½ cylinder for analysis. Group differences between continuous variables were analysed using the analysis of variance (ANOVA) or the Kruskall Wallis test for non-parametric variables. Differences in categorical groups between variables were analyzed using the Chi square or Fischer’s exact test. Pearson’s correlation coefficients were used to identify correlation between continuous variables and these were graphically plotted using the locally weighted scatterplot smoothing (LOWESS) curves. Univariate and multivariable linear regression was used to identify factors predictive of residual stromal thickness and results were presented as coefficient with 95% confidence intervals (CI). In the multivariable models, covariates were chosen if they showed an associated with residual stromal thickness with a p<0.1. The R2 value and regression diagnostics such as multicolinearity, variance inflation and influence of leverage points were used to check the robustness of the regression equations derived. All data was entered in Microsoft Excel and was analyzed using STATA 12.1 I/C (Fort Worth, Texas, USA). All p values <0.05 were considered statistically significant.
6.6 years (median=25 years, IQR= 22 to 28 years). There were 30 women (54%) in the study population and there was an equal distribution of right (n=51, 51%) and left eye (n=49, 49%) undergoing SMILE. The mean preoperative BCVA was 0.03± 0.07 logMAR (range = 0 to 0.3 logMAR) and the mean preoperative spherical equivalent was -5.5D ± 2.05D (median = -5.5D, IQR= ± 29.8 µ (median = 533 µ, IQR= 511 to 552 µ, range = 449 to 629 µ). The pupillary size in the study group was 3.80+ 0.82mm (median= 3.84mm, IQR= 3.3 to 4.3mm).
Forty eyes (40%) had SMILE with a lenticular diameter of 6.5mm, 40 eyes (40%) with lenticular diameter between 6.4 and 6 mm and 20 eyes (20%) with lenticular diameter below 6 mm. All eyes in the 6.5 mm group had SMILE with lenticular diameter of 6.5mm. In the group of eyes that had SMILE with lenticular diameter between 6.4 to 6mm, the mean lenticular diameter size was 6.13+ 0.2 mm (median = 6mm, IQR = 6 – 6.3mm). Distribution of the number of eyes with different sizes of lenticular diameter is shown in figure 1.
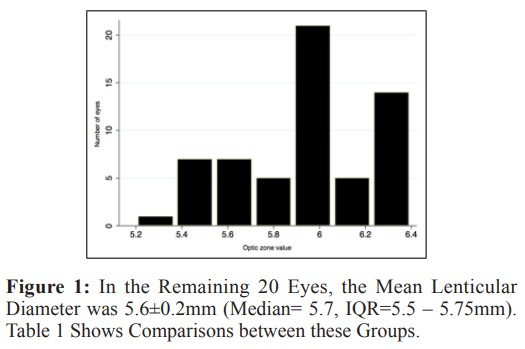
0.2 mm (median = 6mm, IQR = 6 – 6.3mm). Distribution of the number of eyes with different sizes of lenticular diameter is shown in figure 1.
Almost half the patients in all groups reported mild glare and halos at 1 week but there were no differences in contrast sensitivity between groups (Table 2). At 1 month follow up; the UCDVA across groups was 0.02 ± 0.06 logMAR (median = 0 logMAR, range = 0 – 0.3 logMAR). There were no differences in UCDVA and contrast sensitivity between groups with respect to the lenticular diameter (Table 2). However, a higher proportion of patients reported glare and halos in eyes that had SMILE with a lenticular diameter of <6mm (Table 2). The residual stromal thickness was significantly lower in eyes that had SMILE with lenticular diameter ranging between 6.4 to 6mm (Table 2).
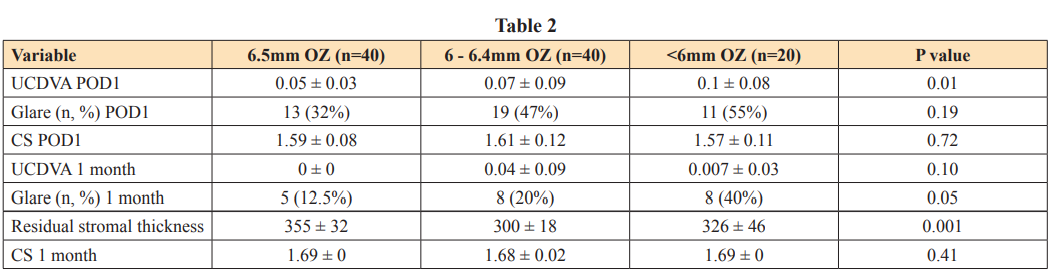
Preoperative pachymetry (r=0.71, p<0.001) and baseline spherical equivalent (r=0.60, p<0.001) had a significant positive correlation with the residual stromal thickness. This relation was linear throughout the range of preoperative pachymetry (figure 2).
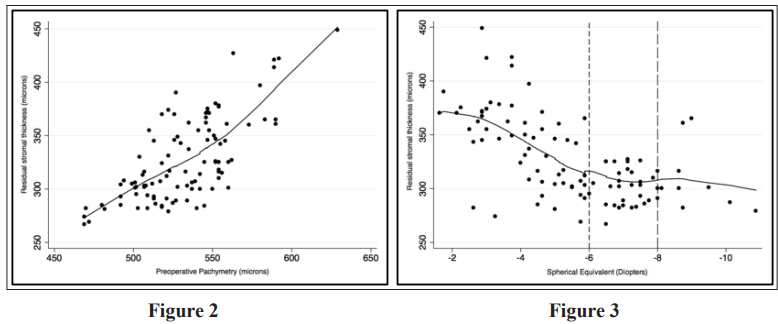
However, a strong positive correlation was seen between residual stromal thickness and spherical equivalent only in the range of -1 and -6 D after which no correlation was weak till -8 D and then no correlation was observed (figure 3). Univariate linear regression analysis showed that age, spherical equivalent, preoperative pachymetry and lenticular diameter size were significantly associated with residual stromal thickness (Table 3).
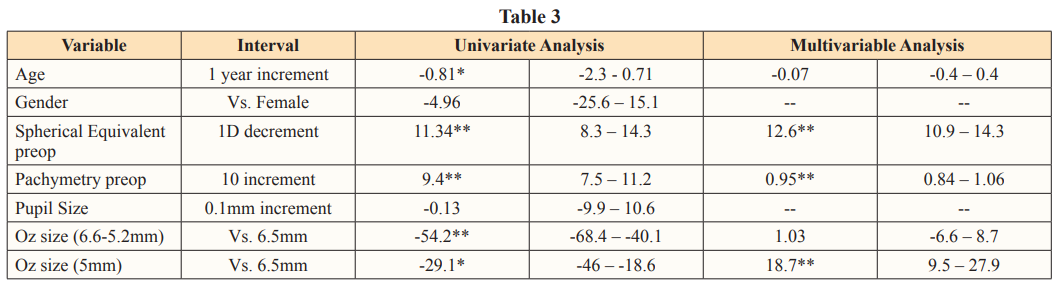
After adjusting for spherical equivalent alone, eyes with <6 mm lenticular diameter had a RST of 11 µ (95% CI= 8.3 – 14.3 µ, p<0.001) greater than eyes with 6.5 mm lenticular diameter for every 1 D increase in myopic refraction. Multivariable linear regression showed that, after adjusting for spherical equivalent and preoperatively pachymetry, eyes with <6 mm lenticular diameter had more than 18 greater residual stromal thickness compared to those with 6.5mm lenticular diameter (Table 3). Eyes with lenticular diameter between 6-6.4mm did not differ significantly in the residual stromal thickness compared to 6.5mm eyes after adjustments with baseline pachymetry and spherical equivalent. The following regression formula was found to accurately predict the residual stromal thickness with a high R2 value of 0.92:

where-113 was the constant, lenticular diameter was 0, 1 or 2 for 6.5mm, 6-6.4 mm and < 6mm respectively, age was in years, spherical equivalent was in diopters and pachymetry was in microns.
In this study, we found that a lenticular diameter of < 6mm lead to excellent refractive correction at 1 month postop with most patients gaining uncorrected vision of 6/6. After adjusting for baseline spherical equivalent and pachymetry, these eyes had 18 µ greater RST compared to eyes with 6.5mm lenticular diameter. Similarly, the RST was 11 greater for every 1D increase in myopic refraction. Patients in the <6 mm group reported marginally greater glare and halos but did not have compromised contrast sensitivity.
In recent times, SMILE is becoming the refractive procedure of choice for treating myopia and myopic astigmatism and is being widely adopted by refractive surgeons globally. Many modifications of SMILE are being presented to improve outcomes and enhance patient satisfaction, many of which have been succinctly enumerated by Tityal et al [13]. One of these modifications is to reduce the size of the lenticular diameter to below the recommended 6.5 mm in an attempt to increase the RST and improve the biomechanical properties of the cornea. Qian et al reported retrospective outcomes from 128 eyes undergoing SMILE using either a 6.5 mm or a 6.2 mm optical zone [14]. Their main outcome measures were corneal power distribution measured by a ray tracing method and functional optical zone obtained via a Scheimpflug camera. Authors reported that the change in total corneal power at 5 mm and 4 mm from the centre of the pupil lead to the most accurate target refraction at 1 month in the 6.5 mm and 6.2 mm groups respectively. These findings prompted us to attempt reduction in the lenticular diameter to as low as <5.2mm. In another study, Fu et al reported outcomes from SMILE performed across three optic zone i.e.> 6.5mm, 6.3 to < 6.5 and <6.3mm [15]. Authors reported good refractive outcomes in all three groups but patients with high myopia (> -6 D) had a larger difference in planned and achieved functional zone compared to those with lower myopia (< - 3 D). Similarly, we found good refractive outcomes in all three groups at 1 month.
Moshirfar et al reported corneal ectasia following SMILE in 7 eyes of 4 patients [11]. In retrospect, all these eyes were seen to have a high Randleman ectasia risk score and the percent tissue altered (PTA) was almost 40% suggesting that these eyes had subclinical keratoconus at baseline. The theoretical advantage of SMILE over femto – LASIK in improving biomechanical strength of the cornea has not been confirmed by many studies [16,17]. Perhaps, it is the residual stromal thickness that is the most important predictor of ectasia than the procedure itself [7]. In our study, we found the RST to be significantly better in eyes with a <6 mm lenticule extracted during SMILE, ranging between 9 and 28 better, compared to those with a 6.5 mm lenticule, after adjusting for the refractive error. Majority of those in the 6.4 to 6 mm group had a lenticular diameter of 6 mm and the RST in this group was no different than those in the 6.5mm group. Based on these findings, we believe that it may be prudent to extract a lenticule with a smaller diameter, especially in eyes with higher refractive error and borderline corneal topography readings.
The increased safety of SMILE afforded by a smaller lenticular diameter may be offset by the greater proportion of patients complaining of glare and halos [15]. In our series, nearly a third of the patients in the >6mm group reported experiencing glare and/or halos. We did not find a significant association between glare and baseline scotopic pupil size or spherical equivalent on statistical analysis (data not shown). Even though there is a small difference between the planned and achieved functional optical zone following SMILE, we believe that our patients, with a mean scotopic pupil size of 3.8mm, may tolerate extraction of a smaller lenticular diameter, especially as this may improve long term safety and prevent ectasia.
The main drawback of the study is the lack of data regarding higher order aberrations induced by the smaller optic zone and the short duration of follow up. To the best of our knowledge, this is the first study reporting on outcomes from SMILE performed with a <6 mm lenticular diameter with a relatively good sample size.
In conclusion, we found that SMILE performed with a lenticular diameter of < 6 mm was effective in delivering excellent refractive correction and improving the corneal biomechanical strength by virtue of significantly greater RST. In view of greater glare and halos, this modification may be best for eyes with higher refractive error, borderline topography suggestive of early keratoconus and scotopic pupillary diameters of less than 4mm.
1.Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A (1990) Laser in situ keratomileusis. Lasers Surg Med 10: 463-468.
2.Schallhorn SC, Farjo AA, Huang D, Boxer Wachler BS, Trattler WB, et al. (2008) Wavefront-guided LASIK for the correction of primary myopia and astigmatism a report by the American Academy of Ophthalmology. Ophthalmology 115: 1249-1261.
3.Farjo AA, Sugar A, Schallhorn SC, Majmudar PA, Tanzer DJ, et al. (2013) Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology 120: e5-e20.
4.Binder PS (2007) Analysis of ectasia after laser in situ keratomileusis: risk factors. J Cataract Refract Surg 33: 1530-1538.
5.Condon PI, O’Keefe M, Binder PS (2007) Long-term results of laser in situ keratomileusis for high myopia: risk for ectasia. J Cataract Refract Surg 33: 583-590.
6.Randleman JB (2006) Post-laser in-situ keratomileusis ectasia: current understanding and future directions. Curr Opin Ophthalmol 17: 406-412.
7.Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD (2003) Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 110: 267-275.
8.Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, et al. (2008) First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg 34: 1513-1520.
9.A?ca A, Demirok A, Y?ld?r?m Y, Demircan A, Ya?a D, et al. (2016) Refractive lenticule extraction (ReLEx) through a small incision (SMILE) for correction of myopia and myopic astigmatism: current perspectives. Clin Ophthalmol 10: 1905- 1912.
10.Ganesh S, Brar S, Arra RR (2018) Refractive lenticule extraction small incision lenticule extraction: A new refractive surgery paradigm. Indian J Ophthalmol 66: 10-19.
11.Moshirfar M, Albarracin JC, Desautels JD, Birdsong OC, Linn SH, et al. (2017) Ectasia following small-incision lenticule extraction (SMILE): a review of the literature. Clin Ophthalmol 11: 1683-1688.
12.Sachdev G, Sachdev MS, Sachdev R, Gupta H (2015) Unilateral corneal ectasia following small-incision lenticule extraction. J Cataract Refract Surg 41: 2014-2018.
13.Titiyal JS, Kaur M, Shaikh F, Gagrani M, Brar AS, et al. (2018) Small incision lenticule extraction (SMILE) techniques: patient selection and perspectives. Clin Ophthalmol 12: 1685-1699.
14.Qian Y, Huang J, Zhou X, Hanna RB (2015) Corneal Power Distribution and Functional Optical Zone Following Small Incision Lenticule Extraction for Myopia. J Refract Surg 31: 532-538.
15.Fu D, Wang L, Zhou X, Yu Z (2018) Functional Optical Zone After Small-Incision Lenticule Extraction as Stratified by Attempted Correction and Optical Zone. Cornea 37: 1110- 1117.
16.Damgaard IB, Reffat M, Hjortdal J (2018) Review of Corneal Biomechanical Properties Following LASIK and SMILE for Myopia and Myopic Astigmatism. Open Ophthalmol J 12: 164-174.
17.Raevdal P, Grauslund J, Vestergaard AH (2018) Comparison of corneal biomechanical changes after refractive surgery by noncontact tonometry: small-incision lenticule extraction versus flap-based refractive surgery - a systematic review. Acta Ophthalmol 97: 127-136.
View PDF