Author(s): Margherita Occhipinti*, F Baccetti, S Bertoli, S Cosimi, Ilaria Casadidio, Ilaria Cuccuro, A Di Carlo, G Gregori, Mary Mori, Emilia Lacaria, Cristina Lencioni, Paola Orsini, Anna Turco and Graziano Di Cianni
Prevalence of type 2 diabetes represent an epidemic problem especially for aging of population. Median age of people affected is rising and Internation Diabetes federation suggest that 1 patients of 5 has more than 65 years. Same situation affected also Italy where in 2020 about 20% of T2D patients were older than 70 years old. This class of patients present often-severe comorbidity as cardiovascular and kidney impairment and are undergoing complex therapies with multiple daily therapy intakes and difficulties in adherence to prescribed therapy. New developed drug, as GLP1-receptor agonist, like Dulaglutide (DU), may be useful in elderly for drug’s ancillary effects and for demonstrated safety on hypoglycemic events. Nevertheless, majority of knowledge, derived from RCT-study, enrolled patients aged 20-65 years and data in elderly patients are limited. Data from real world experience could be useful to understand safety and efficacy of this drug. We retrospectively analyzed data from 751 T2D patients to evaluate DU after 6, 12 and 18 months, comparing people older and younger than 70 years. The introduction of DU, with a relevant number of insulin and sulphanilureas suspensions, statistically reduced HbA1C and body weight after 6 months while glomerular filtration rate (GFR) remained stable and these results lasted over time. About 23% patients dropped-out (8% for gastrointestinal disturbances). No significative differences in tolerability and efficacy, between the two groups were found. DU is a safe, efficacious and easy to use option even for elderly T2D patients.
Worldwide prevalence of type 2 diabetes is increasing and at the same time, median age of population rising, thanks to longevity of population and improvement of care. The International Diabetes Federation report suggest that 1 patients of 5 has more than 65 years. Same situation affected also Italy where, in 2020, about 20% of T2D patients were older than 70 years old. This aged population present often severe comorbidity as cardiovascular and kidney impairment, defining a specially frailty of patients [1, 2].
Simplification of therapy is an increasingly useful goal in the management of chronic disease, especially diabetes. Adherence to therapy is indeed related to the complexity of the treatment regimen and is associated with the achievement of outcomes. Italian data for 2020 say that adherence to therapy does not exceed 30 percent. With this in mind, the choice of treatment options capable of reducing the frequency of administration, glycemic stick checks, reducing the dose of insulin or other therapies burdened by hypoglycemia represent very attractive options [3].
New developed drug, as weekly -GLP1 receptor agonist (GLP1- RA) may be useful in patients older than 65 years especially for safety respect of hypoglycemic event and for ancillary effects. In addition, the ability to simplify therapy is particularly useful for elderly patients, who are often burdened with multiple diseases and undergoing complex treatment regimens. Nevertheless majority of RCT study enrolled patients aged 20-65 years and data in elderly patients are limited [4-12]. Dulaglutide (DU) is a weekly administered GLP1 RA that has already showed, in randomized clinical trials (RCT) superiority vs metformin , sitagliptin, exenatide bis in die, insulin glargine and non-inferiority vs liraglutide in lowering HbA1 C levels High percentages of patients achieved composite outcome of HbA1C < 7 % with no weight gain and no hypoglycemia [4-12]. Moreover, in T2D patients and moderate to severe kidney disease (CKD 3-4 stages), treatment for one year with once weekly dulaglutide showed non inferiority to tritrated insulin glargine (both in add-on to prandial lispro) in ameliorating glycemic control [13]. Beside the significative weight loss and lower rate of hypoglycemia respect to glargine, in the group treated with DU the decline of GFR was mitigated [14]. Further analysis showed no differences in efficacy related to age, gender, diabetes duration and BMI [15-17].
Real world data (RWD) confirm the good safety/efficacy profile of DU and more adherence and persistence to treatment compared to other GLP1ras. Clinical information obtained from both RCT and RWD; suggest the use of DU vs drugs such as sulphanilureas (SU) and insulin especially in people at high risk for the consequences of a hypoglycemia [18]. At the same time, effect on body weight and gastroenteric intolerance represent a possible obstacle to prescription in aged patients due to their frailty, so, after 70 years old, DPP4-inhibitors, represent the most preferred drugs choice for achieve glycemic control. Nevertheless, efficacy of drugs are close concentration-time related, and compliance to the treatment represent an important goal to reach efficacy [3]. Aim of our work was to retrospectively analyze data of our real world experience in 4 diabetologic units of the North of Tuscany with special attention to safety and efficacy in elderly patients.
From February 2016 (launch of DU in Italy) 751 patients (440M/311F) started DU treatment in four diabetologic units of northwest Tuscany. Data were collected till 28 February 2019. In this enrolled population 30% of patients (n. 234) were over 70 years old (mean age 74.5 ±3, 8). DU was prescribed according to parameters of Italian Medicines Agency in add on to oral anti diabetic drugs (OHA) and/or basal insulin or in substitution of OHA and/or insulin. For all patients, parameters of glycemic control i.e. HbA1c, fasting glucose (FPG), weight, BMI, blood pressure (BP) and GFR were collected at baseline (t0) and after mean periods of 6 months (365 patients), 12 months (182patients), 18 months (106 patients) and 24 months (70 patients). Specifically, outcomes in terms of glycemic compensation was evaluated in the population over 70 years old undergoing dulaglutide therapy by assessing the change in treatment regimen
During follow up visits, drop - out reasons (missing data patients, gastroenteric disorders and therapeutic failure) and time of withdrawal (within 1 month, 6 months, 12 months and aftermore than 13 months) were recorded all together and for each group.
Data were retrospectively collected using “MY STAR” clinical data system, (a certified computerized clinical data collecting tool applied in most Italian diabetologic units) uniformally used in every antidiabetic enrolling center.
Uni-variate analysis, using paired and unpaired t test, as properly, were performed to compare results at baseline.
Table 1 report main characteristics at baseline. As expected, proportion of women (47%, p<0, 05 vs Y) and duration of diabetes (p<0,001 vs Y) were significantly higher in the group Older than 70 years old. On the contrary GFR (p<0, 0001) and HbA1c (p<0, 05) at baseline were lower compared to Y group (appendix 1).
| Total N . 751 |
Age < 70 years N. 517 |
Age > 70 years N. 234 |
T test | |
|---|---|---|---|---|
| Age (yrs) | 63,8 ± 9,8 | 59,0 ± 7,6 | 74,5± 3,8 | <0.001 |
| Gender (%) | 440 m/331 f | 317 m/ 200 f | 123 m/ 111 f | 0.025 |
| BMI (kg/m BMI (kg/m2 ) ) | 32,9 ± 6,1 | 33,2 ±5,8 | 32,1±6,5 | 0.02 |
| Diabetes Duration (yrs) | 12,5 ±7,9 | 10,8 ±7,2 | 16,1 ± 8,1 | <0.001 |
| HbA1c before treatment (%) | 8,0 ±1,1 | 8,1 ±1,1 | 7,9 ±1,1 | 0.02 |
Basal therapy before starting DU administration was complex, with at least 43 different combination of drugs reported. At baseline, most of patients (662 patients) were treated with metformin and their number augmented with the use of DU (681patients). The same trend was observed for pioglitazone (98/105 patients). On the contrary, drugs at high hypoglycemic risk such as sulphonilureas SU were reduced (220/146 patients) as well as insulin both basal (149/129 patients) and prandial (65/5 patients). Moreover, before the administration of DU, about half patients were treated with innovative drugs such as DPP4-I (196 patients i.e. 26%) , SGLT2 i (93 patients i.e. 12%) or other GLP1 RA (91 patients i.e 12%) and according to Italian regulatory rules these medications were suspended (appendix 3 ). Even in those patients with sub optimal glycemic control before DU treatment, simplification of therapy was allowed, of course for reduction in number of daily tablets and/or injections but also for number of antidiabetic agents used.
Overall at the first follow-up visit significant reductions of HbA1c ( Δ = 0,9%), fasting plasma glucose (Δ =23mg/dl ) BMI (Δ = 0,9 kg/m 2 ) weight (Δ= 2,4 kg) (all p<0,0001) were observed. Results lasted over time achieved and lasted over time during follow-up visits. Kidney function was preserved during time (GFR 83.4 ± 20 ml/min/1,73m 2 at 6 months; 82, 8 mil/min/1, 73 m 2 after 2 years). Results were comparable for Y and O groups at each follow up visit, too (Figure 1).
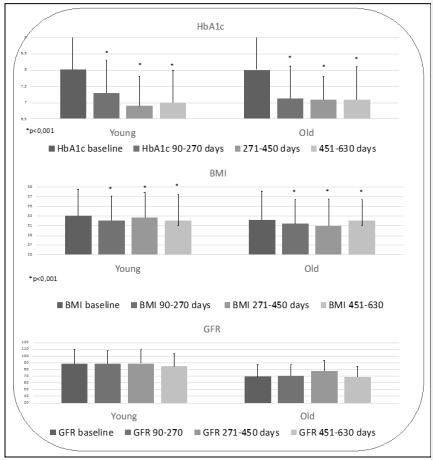
Figure 1: Trends of Glycated Hemoglobin, Fasting Blood Glucose, Blood Pressure and Renal Filtrate before and After Dulaglutide Therapy in the Population above and Below 70 Years of Age
Furthermore, no significative differences in reduction of HbA1c were observed in relation to gender, duration of diabetes (less or more than 10 years) and BMI (<30; between 30 and 35; >35) (Appendix 1).
After the introduction of DU, 172 patients dropped out with similar percentages (22, 5% group Y vs 23% group). Most of patients dropped out (66% of Y and 80% of O) in the first 6 months (81patients first month and 41 patients within 6 months). Overall, in both groups 8% (58 patients) discontinued treatment for gastroenteric problems. Multivariate analysis showed any correlation between failure and age, gender, diabetes duration, FPG, BMI, GFR and any kind of drugs previously used, apart from baseline Hba1c (Figure 2).
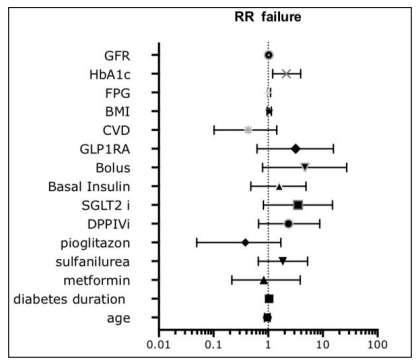
Figure 2: Drop out for Drug Failure on Glycemic Control did not Correlate with any Condition at Baseline Apart from HbA1
This retrospective study, in a real world setting on 751 patients treated with DU confirms the safety/efficacy profile of this medication observed in thousands of patients from RCT. The elderly population, especially evaluated, showed similar beneficial in terms glycemic control, safety and tolerability compared to younger population. Effect on BMI were registered for both younger and older than 70 years old. GFR remained stable during follow-up. We showed that DU is generally well tolerated and dropouts for gastroenteric disturbances were in line with what is known in literature. The results observed were obtained in many types of patients ranging from new-diagnoses to basalbolus insulin treatments. In the elderly group median duration of diabetes was longer than 15 years, confirming effectiveness of DU irrespective of diabetes duration. The introduction of DU allowed in many patients the suspension of drugs at high risk for hypoglycemia such as SU and insulin and in any case, the weekly administration was a simplification of the therapy. No differences were observed for different gender, baseline BMI and age. Recently, more and more work is focusing on simplifying therapy, which does not coincide with deintensification, but rather with reducing the overall impact of the disease on the individual. However, although the need for simplification is shared, the guidelines do not make explicit how to do so. The use of GLP1 agonists, basal insulins combined with GLp1 agonists have been shown in some work to effectively replace complex therapeutic regimens such as the traditional use of basal-bolus insulin therapy [3]. In our experience, the basal bolus regimen was discontinued in 91% of patients despite improved glycemic compensation. Sulfaniureas burdened by high hypoglycemic risk, and particularly in elderly patients, were also discontinued in nearly 65 percent of patients, again with the same glycemic compensation.
These examples confirm in the real world the possibility of simplifying therapeutic regimens by employing effective and safe drugs, with evidence confirmed even in populations older than 70 years.
Limitations of our study include the retrospective nature of the analysis itself, the short duration and the limited number of patients who underwent the follow-up visits. Do to the short follow up no data on cardiovascular events were collected. Similarly, in what report on literature and even in what observed in REWIND trial, about 30% of patients enrolled had a previous established cardiovascular event report.
Nevertheless first observations reported in our work suggest that we have another opportunity to efficacy treat diabetes in elderly patients with simplification of therapeutically-scheme and avoiding high-risk hypoglycemia agents use in aged patients as in younger.
