Author(s): Fayzieva Nozima, Mailo Janette, Rajapakse Thilinie, David Barry Sinclair, Bhargava Ravi and Kassiri Janani*
Febrile seizures are common in children, with incidence rates up to 14% in developing countries; febrile status epilepticus accounts for roughly 5% of all febrile seizure cases. Both status epilepticus and febrile status epilepticus are life-threatening conditions that put children at risk of future epilepsy and that require timely diagnosis and care. However, there are difficulties in applying these diagnostic concepts to daily practice due to a lack of precise definitions of the disorders. In addition, there are no unified standardized neuroimaging diagnostic guidelines, and the significance of imaging findings, when observed, remains uncertain. Even though some brain MRI features occurring in the acute phase and over long-term follow-up have been studied in both children and animals, the causal relationships between these findings and risk of adverse seizure outcomes still need clarifying. Guidelines on eligibility criteria, optimal imaging modalities and protocols, timing of imaging, and the specific brain areas and structures to be evaluated and reported, along with their specific characteristics, are urgently required. This review summarizes clinical issues related to the varied definitions of febrile status epilepticus and the data regarding the acute and chronic neuroimaging changes observed in febrile status epilepticus.
Febrile seizures (FS) are defined as seizures occurring in febrile children aged between 6 and 60 months who do not have an intracranial infection, metabolic disturbance, or history of afebrile seizures [1]. FS are common, affecting 2-5% of children in the United States and Western Europe, 6% to 9% of children in Japan, and 14% of children in India and Guam [2]. Status epilepticus (SE) affects ~20 children per 100,000 each year and is defined by the 2012 Neurocritical Care Society as a seizure with 5 minutes or more of continuous clinical and/or electrographic seizure activity, or recurrent seizure activity without recovery between seizures [3]. Of children suffering from convulsive SE, about a quarter have febrile status epilepticus (FSE), a medical emergency that can be defined as a seizure of prolonged duration (>30 minutes) in a febrile child. FSE develops in 5% to 9% of patients who experience an initial FS [4, 5].
However, there are issues with these definitions, particularly with respect to the length of seizure duration, age of onset, and brain maturity. For instance, there have been different definitions of SE over the last 40 years, the most widely recognized, based on animal studies, as being a 30-minute period of seizures [6]. Later, the literature has popularized new terms like early or impending
SE, which introduce the concept of 5-7-minute-long seizures and allows earlier medical intervention [7-11]. Similar timing- related issues apply to FSE. While a decrease in seizure duration from 30 to 5 minutes as a definition for FSE could make the definition more clinically practical, it creates additional complexity in understanding the difference between a simple versus complex seizure, which still includes a 15-minute threshold.
With respect to age and brain maturity, the minimum age of seizure onset for a definition of FS differs according to definition and is either 1 or 3 or 6 months of age [1, 2, 7 & 12]. The six-month threshold is problematic since, if seizures occurring before 1 month of age are considered neonatal seizures and FS start at 6 months, this creates uncertainty about seizures occurring between 1 and 6 months. Moreover, in children younger than 6 months, a seizure event can be associated with an infectious etiology such as viral meningitis, so work-up and management are different [13, 14].
While FSE is thought to represent the extreme end of the complex FS spectrum, it is apparent that seizure duration is not the only factor to consider. The young age of seizure onset, immaturity of the brain, and prolonged seizure activity are the aspects of concern in regards to further seizure consequences [15].
FSE is a subtype of both FS and SE. FSE appears to have worse outcomes than simple FS but better outcomes than CSE [16].
The clinical sequelae of FSE are heterogeneous but it is unlikely to result in death or serious morbidity. With the exception of developing countries, there were no documented cases of febrile seizure-related deaths on record [17]. There is some evidence of hippocampal and memory deficits in FSE. Neuroimaging would be useful to both diagnostically and prognostically if salient features could be defined.
The objective of this review is to provide an overview of FSE and review the acute and chronic imaging changes seen in FS and FSE patients.
Animal models of FSE are only an approximation of the human FSE. Research into the temporal relationship between FSE and the development of epilepsy has also been undertaken in rats, mice, and primates [18-23]. FSE has been modeled in rodents and primates via different heating methods to study its clinical features, EEG findings, and subsequent development of afebrile seizures. Studies of imaging features predicting risk of seizure consequences were mainly conducted in rats. While the results from animal models were promising, the use of ultrahigh field strength scanners, initial imaging within an ultrashort time (?2 h) after FSE, serial imaging within a short time interval, similar age and baseline conditions of case subjects within the group and with controls, and the short latency period between FSE and the development of adverse consequences have made it difficult to replicate the same results in humans.
Nevertheless, imaging findings such as asymmetry in T2 relaxation times between the left and right hippocampus and amygdala and unilateral reduction of T2 relaxation times were observed in rats with FSE that became epileptic. T2 values in the basolateral and medial amygdala and medial thalamus predicted epileptogenesis better than chance [19]. Several MRI scans repeated over time within 2 to 48 hours improved prediction of epileptogenesis compared with a single MRI scan. The T2 difference in the medial amygdala was a better predictor of epilepsy than that in the basolateral amygdala [20]. According to the results of whole-brain T2 changes, rats with experimental FSE (eFSE) were classified as eFSE-vulnerable or eFSE-resilient; eFSE-vulnerable rats were found to have a minimal reduction in T2 relaxation times, which was significantly different to that observed for control or eFSE- resilient rats. The prolongation of T2 relaxation times in whole brains, the hippocampus, and the basolateral amygdala was also found to predict worse cognitive outcomes in rat models [18].
Prolonged FS or FSE has been associated with the subsequent development of afebrile seizures, epilepsy and/or hippocampal atrophy, and the nature of these associations remains ambiguous [15, 24-26]. While not all patients with FSE develop further complications, it remains unclear what specific characteristics define patients at risk for developing epilepsy.
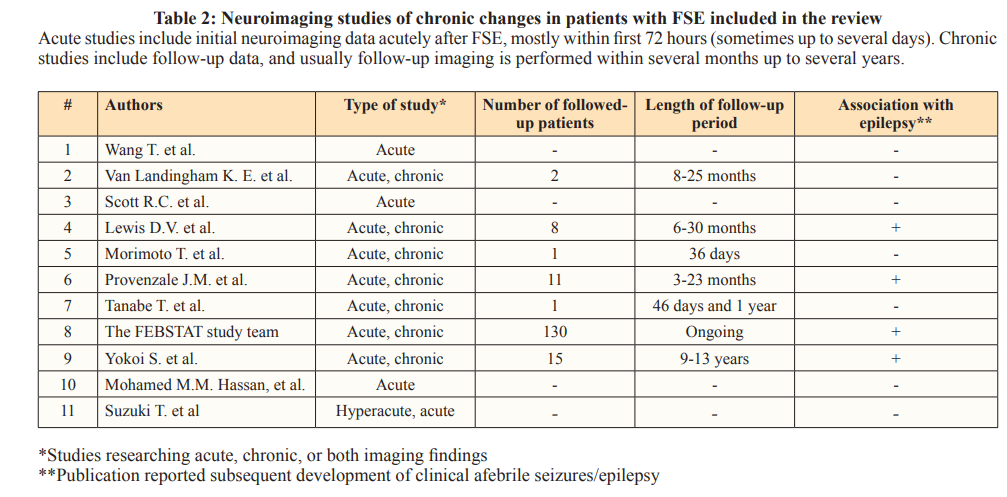
Several studies have evaluated prognostic value of imaging, particularly MRI, to detect features that might identify children at a higher risk of adverse outcomes from FSE, such as afebrile epilepsies including temporal lobe epilepsy (TLE) and/or medial temporal sclerosis (MTS) (Tables 1 and 2). Wang et al. presented one of the first cases of MR imaging of a child with SE meeting the criteria for FSE [27]. In their report, while CT showed only a choroidal fissure cyst, MRI was more sensitive and detected increased T2 signal and enlargement of the entire right hippocampal formation. Van Landingham et al. attempted to identify signs of acute brain injury shortly after a seizure, particularly in the temporal lobe structures [28]. They reported that MRI abnormalities were only apparent in patients with focal or lateralized features. MRI changes suggestive of acute edema were observed in patients with significantly longer seizures, and two patients with initial MRI abnormalities showed hippocampal atrophy on follow-up MRI. A subsequent study re-examined the patients recruited to the study prospectively and whose first imaging was within 72 hours of the initial seizure, with permanent brain injury the outcome of interest. The initial MRI scans showed increased T2 signal shortly after prolonged FS and increased hippocampal volume. All abnormalities were unilateral and located in the hemisphere of seizure origin based on clinical localizing signs. Follow-up scans taken several months later showed that hippocampal volume decreased markedly and the T2 signal remained abnormal [29]. The same group went on to report a strong association between MRI evidence of a markedly hyperintense hippocampus in FSE children who subsequently developed MTS [30]. Conversely, Tanabe et al. revealed acute and follow-up MRI changes compatible with previous reports in only one patient out of 59 with FSE, concluding that the prevalence of hippocampal injury after FSE may be in the range of 0 to 10% [31].

CFS – complex febrile seizure, FS – febrile seizure, SE – status epilepticus, CFC – complex febrile convulsion, PFC – prolonged febrile convulsion, CNS – central nervous system, FC – febrile convulsion, FSE – febrile status epilepticus, PFS – prolonged febrile seizure.
A case report of sequential brain CT and MRI in a child with FSE and previously normal neuroimaging showed transient and harmless acute cytotoxic brain edema but irreversible delayed brain edema with hyperintense signal changes on T2-weighted images [32]. The patient subsequently developed atrophy and high signal on T2-weighted images, suggestive of gliosis.
FEBSTAT was a large prospective study to determine whether FSE generates acute hippocampal injury that develops into hippocampal sclerosis (HS). In children with FSE, 22 out of 199 had acute increases in hippocampal T2 signal after FSE associated with increased volume; these changes were not observed in any of the 96 simple FS control subjects [33]. Follow-up MRIs in 14 of these 22 patients showed that 10 and 12 patients had HS and reduced hippocampal volume, respectively. By contrast, follow-up of 116 children without acute hyperintensity revealed an abnormal T2 signal in only one patient, who had experienced another episode of FSE. Hippocampal malrotation was seen in 20 subjects in the FSE group compared with two control subjects [34-36]. It was uncertain whether these MRI abnormalities were present before injury and predisposed for injury or whether they occurred after injury. Among the 22 patients with an abnormal T2 signal on the initial MRI, four had hippocampal malrotation. The percentages of extrahippocampal abnormalities in the two groups were similar. However, extrahippocampal abnormalities located in the temporal lobe were more common in the FSE group. Furthermore, compared to control subjects, FSE subjects with normal acute MRIs had abnormally low right-to-left hippocampal volume ratios, initially smaller hippocampi, and reduced hippocampal growth. At the time of reporting, 16 patients with FSE had developed epilepsy, and mostly not TLE, while five of these patients were among the 22 subjects with initial T2 hyperintensity [5].
Yokoi et al. reported that the hippocampal hyperintensity on T2- weighted images was consistent with the hyperintensity of the same region on diffusion-weighted imaging (DWI) in children with FSE [37]. Unilateral hippocampal hyperintensity was present in 27% of patients evaluated on initial imaging, with follow-up data obtained between 9 and 13 years after initial imaging. All patients with hippocampal hyperintensity developed focal epilepsy and had signs of hippocampal atrophy, compared with only 1% without initial hippocampal hyperintensity. Suzuki et al. also found evidence of cytotoxic edema in the cortex on hyperacute diffusion-weighted imaging (DWI) before the appearance of any hippocampal abnormalities [38]. However, the authors concluded that edema was not associated with unfavorable short-term outcomes.
Hassan et al. used magnetic resonance spectroscopy (MRS) to determine hippocampal metabolic changes in patients with prolonged FS [39]. The authors found that MRS was a highly sensitive predictor of TLE, even in patients without any MRI abnormalities, and MRS revealed hippocampal injury more accurately and earlier than EEG. However, larger studies with longer follow-up are required to establish the association between these early metabolic changes and subsequent development of TLE.
Current Bottlenecks to Developing Neuroimaging Guidelines for FSE
There are currently no neuroimaging guidelines to help decide which FSE patients should be imaged, when they should be imaged, which imaging modality and protocols should be used, which areas of the brain should be particularly examined,
what significant findings should be reported, who should have longitudinal imaging, and when follow-up imaging should occur. Retrospective studies have demonstrated that not all patients with FSE are routinely imaged, that different imaging modalities and protocols are used, and that the time of imaging is mostly based on either the clinical course of the disease or availability of imaging tools in individual centers. Moreover, imaging is often performed to exclude other pathologies rather than search for findings compatible with FSE, so the scans are usually reported as normal.
Neuroimaging is an essential assessment in pediatric patients with epilepsy. SE studies prioritize four non-mutually exclusive factors: diagnosis, localization, evaluation of pathophysiology, and prognostication [40]. CT and MRI are most often used to evaluate patients with SE, with CT used more widely than MRI due to shorter imaging times and wider availability [41]. However, MRI is believed to be more sensitive and specific for the detection of subtle changes in the hippocampus [27]. Challenges to MRI in children include the need for sedation and difficulties in interpretation due to age. If a child cannot remain motionless long enough to obtain high-resolution images, the approved sedation method is general anesthesia. Separately, ongoing myelination process over the first 2-3 years of life can make MRI interpretation difficult. Between 6 and 18 months of age, the phase of signal reversal may lead to false-negative results when detecting epileptogenic lesions [42].
While 1.5 T field strength MRI is routinely used in clinical practice, a 3.0 T field strength is preferable to enhance the signal-to-noise ratio, spatial resolution, and reduce scan times. Furthermore, 3.0 T scanners are superior to 1.5 T scanners for recognizing minute, hemosiderin-containing, or calcified lesions. However, 3.0 T MRI has some theoretical limitations, as high radiofrequency energy deposition can cause unpleasant heating of the patient’s body, which can be partially overcome by parallel data acquisition [40]. Parallel data acquisition techniques may appear noisier, but specific regions of interest may be better depicted due to a reduction in artifacts [42]. Additionally, higher field strength MRI machines are not readily available in every emergency unit due to their higher costs.
The Society for Pediatric Radiology Neuroradiology committee recommends that routine MRI sequences for brain seizures should include T1 3D in the sagittal plane, T2 fluid-attenuated inversion recovery (FLAIR) in the coronal plane, T2 fat-saturation fast spin echo in the coronal and axial planes, T2 axial fast spin echo, axial susceptibility-weighted imaging, and axial diffusion [43]. Optional sequences are available based on specific indications. In the late 1990s, The International League against Epilepsy recommended using temporal angulation for the examination of epileptic children, because patients primarily had temporal lobe epilepsies. The temporal angulation sequence is along or perpendicular to the hippocampal long axis, improving the visualization and evaluation of the hippocampus and temporal lobe [44]. More recently, an increase in the percentage of patients with subtle cortical abnormalities located primarily in the dorsal frontal and parietal lobes led to the recommendation of a FLAIR sequence with anterior–posterior commissure angulation [42].
Some brain structures such as the hippocampus and amygdala can be assessed scrupulously using quantitative MRI [45]. Some subtle changes, such as an increase/decrease in size or evidence of hippocampal asymmetry, may be detected using quantitative MRI, even in patients with FSE who show no apparent structural abnormalities. A decrease in hippocampal volume and an increase in hippocampal signal intensity on T2-weighted images are defined MRI parameters for HS [46, 47]. Additionally, changes indicating cytotoxic and vasogenic edema in children with SE have been identified utilizing DWI and the apparent diffusion coefficient (ADC) [6].
The definitions of FSE are not used consistently in the published literature, and usually seizures meeting criteria for FSE are defined as something else. Only studies published in English were included in the review. Several studies claiming to include children with prolonged FS did not clearly indicate the length of seizures, which could bias the diagnosis and interpretation. Due to a lack of standardized neuroimaging guidelines and rapid changes in imaging techniques and technologies, various MRI protocols have been used over time, making comparisons difficult and compromising the generalizability of the results. Not all studies included long-term follow-up data, and there was a lack of high- quality evidence supporting an association between MRI findings and further seizure consequences.
Children admitted with FSE may show increased T2 signal intensity in the hippocampus by MRI, an increase in hippocampal size due to cytotoxic edema, and hippocampal malrotation. While acute brain edema seems to be transient and harmless, the significance of the association between increased T2 signal intensity and hippocampal malrotation and seizure activity remains unclear. Studies that included follow-up imaging showed persistent increases in T2 signal, which may be a sign of gliosis or HS; hippocampal atrophy or a decrease in hippocampus size; and delay in hippocampal growth compared to controls. Patients with increased T2 signal intensity will also have increased signal in hyperacute DWI. MRS seems to be a promising modality for the early detection of seizure- related metabolic changes, but larger studies with prolonged follow-up are necessary to evaluate the prognostic value of these findings. Animal studies have demonstrated the predictive value of asymmetry in T2 relaxation times in various brain areas and changes in the reduction in T2 relaxation times over time, but these findings need to be replicated clinically.
Unified standardized neuroimaging guidelines are urgently needed for the evaluation of patients with FSE. These guidelines should include criteria defining the patients who need to be imaged, the optimal imaging modality, imaging protocols, timing, and the specific brain areas and structures to be evaluated and reported, along with their characteristics.
Studies reporting imaging findings in FSE and those comparing complex versus simple FS employed different inclusion/exclusion criteria, so comparing these results may not be appropriate. Larger prospective studies with detailed attention to the hippocampus are necessary to establish exactly which features in early MRI are associated with long-term outcomes in children with FSE. Moreover, due to changes in the definitions of SE and FSE, seizures lasting between 5 and 30 minutes should be accounted for in SE and FSE.
