Author(s): Aamir Jalal Al Mosawi
It was known earlier that although, SARS-CoV was controlled more than a decade ago, more SARS-like coronaviruses which have been isolated in bats can use the human angiotensin converting enzyme-2 (ACE2) receptor to replicate to high levels in primary human airway, and they had the potential to cause new epidemics. Now, we should remember that it was concluded from earlier experiences that the first and most effective line of defense against a new viral pandemic in the early phases, are antiviral drugs not vaccines developed after rather a lengthy time. The unexpected high mortality associated with the ongoing SARS-CoV-2 in countries like the United States and the United Kingdom confirmed the earlier scientific prediction of the inadequate preparedness of the world to victoriously combat a new virus pandemic. The rational scientific approach to face a potentially fatal viral pandemic with no known effective specific therapies dictate the early use of all the useful preliminary research evidence with prioritizing emphasis on safety to avoid making more harm than good in such situation. We have previously published an evidence-based keys to the therapeutic challenge associated with the emergence of SARS-CoV-2 pandemic. The aim of this paper is to provide and important updates to keys to the therapeutic challenge which are hoped to help in cracking the padlock of SARS-CoV-2.
Influenza A virus pandemic (1918-1919) was the most deadly pandemic in modern history of medicine, and was caused by type A/H1N1. Influenza a virus can also cause disease in birds and mammals, and it is the chief circulating widely spread virus in the humans. Thereafter, Influenza viruses allowed another virus to dominate the scene during the 21st century. The 2009 swineoriginated virus (pH1N1) pandemic should have informed the scientific of the inadequate preparedness of the world to victoriously combat a new influenza strains pandemic or other new viruses pandemic. Viral pandemics associated with the emergence of new viruses can early defeat the world before vaccine development efforts yield any useful fruit. By now the world should have already declared that the new bat-human corona-virus has been the winner. Waiting for the time when, a vaccine can be used as a main weapon to fight a new viral pandemic is expected to be associated early defeat and too many losses [1, 6].
It was known earlier that although, SARS-CoV was controlled more than a decade ago, more SARS-like coronaviruses which have been isolated in bats can use the human angiotensin converting enzyme-2 (ACE2) receptor to replicate to high levels in primary human airway, and they had the potential to cause new epidemics [1-4]. Now, we should remember that it was concluded from earlier experiences that the first and most effective line of defense against a new viral pandemic in the early phases, are antiviral drugs [1-6].
There are four genera of coronaviruses including alphacoronaviruses, beta-coronaviruses, gamma-coronaviruses, and delta -coronaviruses. Alpha and beta-coronavirus can infect mammals, while gamma-coronavirus and delta-coronavirus generally infect birds. Four coronaviruses are known to cause mild upper respiratory infection in humans of all ages including infants. The transmission of coronaviruses from animals (birds) to causes respiratory illness has been reported as early as 1969 by Kapikian et al. Community-wide outbreak associated with 229E-like coronavirus has be reported as early as 1970 by Cavallaro and Monto. Until December, 2020, two beta-coronaviruses SARS coronaviruses and MERS-coronaviruses were known to cause severe, potentially fatal pneumonia-like illness [1-6].
Errors in the replication of viral genomic RNA of zoonotic coronaviruses led to the emergence of genetically related diverse quasi-species, while the transmission of some of them to a new host species led to the emergence of human severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). SARS-CoV emerged for the first time in Guangdong China in 2002 spread rapidly to many other counties causing more than 8000 cases with about10% mortality. In 2012, it was thought that MERS-CoV was transmitted to humans from bats through an intermediate camel host leading to 1700 cases in 27 countries with about 40% mortality. Increasing number of cases of severe potentially fatal pneumonia caused by a new β-coronavirus was reported from Wuhan China in December 2019, and human-to-human transmission was confirmed early. On the 12 th of January, 2020 the
World Health Organization (WHO) officially named the condition coronavirus disease 2019 (COVID-19). The Coronavirus Study Group of the International Committee suggested naming the new coronavirus “SARS-CoV-2” [1-4].
According to the live online update available at https://www.worldometers.info/coronavirus/, on the 17 th of June 2020, more than 8.18 million cases have been reported from 188 countries and territories. A total of 443,000 deaths have been resulted, while more than 3.96 million cases have recovered.
On the 26th of March, 2020 the United States has become the country with the world’s third largest population, it surpassed China and Italy as the country with the highest number of confirmed cases in the world.
On the 27th of May 27, 2020, the United States had the most confirmed active cases and deaths in the world, and on the fifth of June, 2020, the United States death rate was 330 per million people, the ninth-highest rate globally.
On the 16th of June, 2020, there have been 298,136 confirmed cases in the United Kingdom and 41,969 of confirmed cases died, and United Kingdom had the world’s second-highest death-rate per capita. More than 90% of the patients died had underlying medical condition or were over 60 years old.
The unexpected high mortality associated with the ongoing SARSCoV-2 in countries like the United States and the United Kingdom confirmed the earlier scientific prediction of the inadequate preparedness of the world to victoriously combat a new virus pandemic. In Iraq, the spread of SARS-CoV-2 was first recognized during February, 2020 with the report of the first confirmed cases of SARS-CoV-2 infections on the 22 nd of February in the province of Najaf. The first reported case in Iraq was an Iranian student of religion. The first case SARS-CoV-2 in the Kurdish region of Iraq in the North of the country was reported on the first of March, 2020. However, the first death from SARS-CoV-2 in Iraq was reported from the province of Sulaymaniyah in the Kurdish region of Iraq. The patient was a 70-year-old man who was known to have chronic heart failure associated with asthmatic manifestations. The second death in death from SARS-CoV-2 in Iraq was reported from the province Baghdad, while the first recovery was reported on the 6th of March. On the 27th of March,2020, all 19 Iraqi provinces cases have reported confirmed cases, and on the 7th of April, 28,414 tests have been performed with 1202 of them were positive. On the first of April, 2020, the total number of confirmed SARSCoV-2 cases in Iraq was 728. Early during June, the number of the reported cases exceeded 12,000, and the deaths exceeded 300.
| Province | Cases | Deaths | Recovered |
|---|---|---|---|
| Baghdad | 2,234 | 97 | 941 |
| Al-Anbar | 6 | 0 | 5 |
| Al-Qadisiyia | 15 | 1 | 11 |
| Babil | 49 | 5 | 39 |
| Basra | 747 | 18 | 578 |
| Thi Qar | 96 | 4 | 72 |
| Diyala | 45 | 5 | 21 |
| Duhok | 102 | 0 | 26 |
| Erbil | 397 | 4 | 243 |
| Halabja | 25 | 0 | 22 |
| Karbala | 152 | 8 | 118 |
| Kirkuk | 72 | 2 | 59 |
| Maysan | 52 | 2 | 45 |
| Muthanna | 117 | 4 | 95 |
| Najaf | 431 | 6 | 324 |
| Nineveh | 12 | 0 | 6 |
| Suleimaniya | 811 | 23 | 246 |
| Saladin | 127 | 0 | 18 |
| Wasit | 1,018 | 18 | 139 |
| Total | 12,366 | 346 | 5,168 |
In Iraq, a high SARS-CoV-2 ministerial committee was established by the Iraqi Ministry of health to lead the efforts to control. The committee designed an official protocol (Figure-1A) for the treatment of SARS-CoV-2 and distributed it to hospitals. Lopinavir/ritonavir + ribavirin combination was at the top of the suggested therapies to be used in the treatment of SARS-CoV-2, and that was excellent from the evidence-based medicine point of view. However, the suggested treatment was not available at all in the country. The second suggested treatment was plasma therapy which it was impossible to offer to any patient needs treatment supposing that is an acceptable therapeutic option.

Figure 1A: The Official Protocol Designed By the Higher Ministerial Committee for the Treatment of Sars-Cov-2
Lopinavir/ritonavir + ribavirin combination was also at the top of therapies to be used in the treatment of SARS-CoV-2 in the protocol suggested by Saudi Arabia Ministry of Health (Figure1B). The second treatment option was Favipiravir which was supported by unpublished reference [Reference-31. A Trial of Favipiravir and Hydroxychloroquine combination in Adults Hospitalized with moderate and severe Covid-19 CLINICAL TRIAL PROTOCOL, King Abdullah International Medical research Center, Protocol V1 date April 5 th 2020] (Figure-1C).
The SARS-CoV-2 is an enveloped non-segmented positive-sense RNA virus β-coronavirus (subgenus sarbecovirus, Orthocoronavirinae subfamily) with 96.2% of its genome is identical to a bat coronavirus RaTG13, and 79.5% of its genome is similar to SARS coronavirus. Therefore, bats, the natural host of many of the coronaviruses including SARS-CoV-like and MERS CoV-like viruses, are the most likely natural host of SARS-CoV-2, which was possibly transmitted to a human an intermediate host. SARS-CoV-2 is likely using angiotensin-converting enzyme-2 (ACE2) to infect humans. [1-4,7]. Table-2 summarizes the common features of SARS-CoV-2 that occurred in 55,924 cases in China and reported by Max Roser and Hannah Ritchie in February, 22, 2020. Figure 2 shows a sketch of SARS-CoV-2 appearance with electron microscope.
| Fever | 87.90% |
| Dry cough | 67.70% |
| Fatigue | 38.10% |
| Production of sputum | 33.40% |
| Shortness of breath | 18.60% |
| Muscle or joint pain | 14.80% |
| Sore throat | 13.90% |
| Headache | 13.60% |
| Chills | 11.40% |
| Nausea and vomiting | 5% |
| Nasal congestion | 4.80% |
| Diarrhea | 2.70% |
Studied the CT findings of sixty seven patients with SARS-CoV-2 [8]. Three patients (4.5%) had mild illness, 35 (52.2%) were considered ordinary cases, 22 (32.8%) were considered to have severe illness, and seven (10.4%) were patients critically ill. The three mild cases had no abnormality on CT-scan. In the 35 ordinary cases, three patients had single lesions, 33 patients, multiple lesions, one patient with severe illness had single lesion, and 21 patients had multiple lesions. CT findings of ordinary cases included mainly solid plaque shadow and halo sign (18/35, 51.4%). Fibrous strip shadow with ground glass shadow was more frequent in patients with severe illness (7/22, 31.8%). Consolidation shadow as the main lesion was observed in 7 cases, and all of them were severe or critical ill patients. Zhong et al thought that CT findings of solid shadow may predict severe and critical illness.
Reported a cross-sectional multicenter study conducted in ten hospitals in Hubei province which included 25 confirmed pediatric cases of SARS-CoV-2 with a median age of three years [9]. Ten patients (40%) of the patients of Zheng et al aged less than three years. The most common symptoms at onset of illness were fever which occurred in 13(52%), dry cough occurred in 11 (44%) patients. Chest-CT didn’t show significant abnormality in eight patients (33.3%). Unilateral involvement of lungs was found five (20.8%) on chest CT-scan and bilateral involvement was found eleven patients (45.8%).
Clinical diagnoses included upper respiratory tract infection in eight patients, mild pneumonia fifteen patients, and two patients were considered critical cases. The two critical cases were treated with invasive mechanical ventilation, corticosteroids, and immunoglobulin. The symptoms in 24 patients (96%) of 25 patients improved and one patient was discharged. The work of Zheng et al suggested that children are also susceptible to SARSCoV-2 like adults, but their clinical presentations and outcomes were more favorable than in adults. Zheng et al emphasized that children less than three years old accounted for large number of pediatric cases and critical cases were in this age group. The rational scientific approach to face a potentially fatal viral pandemic with no known effective specific therapies dictate the early use of all the useful preliminary research evidence with prioritizing emphasis on safety to avoid making more harm than good in such situation. We have previously published an evidence-based keys to the therapeutic challenge associated with the emergence of SARS-CoV-2 pandemic [1-4]. The aim of this paper is to provide and important updates to keys to the therapeutic challenge.
Bat-Human Coronaviruses: An Update to Keys to the Therapeutic Challenge Until now, little evidence is available that can support a specific antiviral treatment for SARS-CoV-2. Antiviral drugs (oseltamivir, peramivir, zanamivir, ganciclovir, acyclovir, ribavirin monotherapy) are better to be avoided based on the previous experiences with treatment of coronaviruses [1-4].
The first SARS coronavirus infection was causing respiratory failure in about 20% of patients; therefore, empiric treatments were justified including protease inhibitors (Lopinavir/ritonavir) in combination with ribavirin. Treatment of coronaviruses suggested that Lopinavir and ritonavir, protease inhibitors improved the outcome of MERS-CoV and SARS CoV patients. Chu et al (2004) reported the treatment of 41 patients with SARS with a combination of Lopinavir/ritonavir and ribavirin. The clinical and virological outcomes were compared with 111 patients treated with ribavirin monotherapy. Poor clinical outcomes including acute respiratory distress or death were significantly lower in the Lopinavir/ritonavir treated patients. The experimental work of Chan et al (2015) showed that treatment with Lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. Park et al (2019) studied the efficacy of ribavirin and Lopinavir/ritonavir as Post-exposure prophylaxis for healthcare workers exposed to patients with severe MERS-CoV pre isolation pneumonia. They found that therapy was associated with a 40% decrease in the risk of infection without the occurrence of severe adverse effects. Beta-coronavirus viral loads of a SARS-CoV-2 in a Korean patient were markedly reduced following Lopinavir/ritonavir treatment, while combined treatment with Lopinavir/ritonavir, arbidol, and Shufeng Jiedu Capsule (SFJDC, a traditional Chinese medicine) was associated with improvement in pneumonia caused by SARSCoV-2 [10-15].
Figure 2: shows the chemical structure of Lopinavir/ritonavir. Table 3 summarizes the information relevant to the clinical use of Lopinavir/ritonavir.
Figure 2: A Sketch of the Chemical Structure Lopinavir/ Ritonavir
| Lopinavir/ritonavir (Kaletra) is a preparation with fixed dose Lopinavir and a low dose ritonavir developed for the treatment and prevention of HIV/AIDS. The main action of ritonavir is slowing down the breakdown of Lopinavir. |
| Approval and value |
|---|
| The preparation Lopinavir/ritonavir was approved in the United States in 2000 and it is on the World Health Organization’s List of Essential Medicines which is the list of the safest and most effective medicines needed in a health system [33]. |
| Administration |
| Oral as a tablet, capsule, or solution. |
| Common side effects |
| Diarrhea , nausea , and vomiting especially in children Tiredness and asthenia Headaches Muscle pains |
| Possible serious side effects |
| Pancreatitis Liver injury Hyperglycemia |
| Use during pregnancy |
| Probably safe, and use is common. |
| Use with caution in |
| Structural heart disease Cardiac conduction abnormalities Ischaemic heart disease Cardiomyopathies |
Remdesivir (GS-5734) is a nucleotide analog which was developed for the treatment of Ebola virus disease and Marburg virus infections. It attacks viral RNA-dependent RNA polymerase (RdRp) and causes in premature termination of viral RNA transcription. It can show antiviral activity against single stranded RNA viruses including respiratory syncytial virus [1-3].
Sheahan et al (2017) reported that Remdesivir can inhibit SARS-CoV and MERS-CoV replication in multiple in vitro systems, including primary human airway epithelial cell cultures with submicromolar IC 50 values. According to Sheahan et al, Remdesivir was also effective against bat coronaviruses, pre pandemic bat coronaviruses, and circulating contemporary human CoV in primary human lung cells suggesting that Remdesivir has broad-spectrum anti-CoV activity. Sheahan et al also reported that in a mouse model of SARS-CoV early treatment with Remdesivir significantly reduced lung viral load and improved clinical signs of disease and respiratory function. Agostini et al (2018) reported that Remdesivir effectively inhibits human and zoonotic coronaviruses in vitro and in SARS-CoV mouse model. They showed that Remdesivir inhibits murine hepatitis virus (MHV) with similar 50% effective concentration values (EC50) as SARS-CoV and MERS-CoV. Brown et al showed that Remdesivir has effective antiviral activity against endemic human Coronaviruses OC43 (HCoV-OC43) and 229E (HCoV-229E) with submicromolar EC50 values. They emphasized that the delta coronavirus genus have the most divergent RdRp as compared to SARS- and MERS-CoV and both avian and porcine members harbor a native residue in the RdRp that confers resistance in beta-coronaviruses. However, Remdesivir is highly effective against porcine delta coronavirus. Remdesivir was used in the treatment of the first patient with COVID-19 in the USA and treatment was considered successful and probably stopped for the progression of pneumonia [16-19]. Figure 3 shows the chemical structure of Remdesivir.
Figure 3: A Sketch of the Chemical Structure of Remdesivir
The suggested dose of Remdesivir is 200 mg on the first day, followed by 100 mg once daily. Possible side effects include nausea, vomiting, rectal hemorrhage, and hepatic toxicity.
Chloroquine was thought to have an effect on SARS-CoV infection and spread which can be attributed to immunomodulatory effects, suppression of the production/release of TNF-α and IL-6, autophagy inhibition, and interference with the glycosylation of cellular receptors of SARS-Co. Chloroquine may act on entry and at post-entry stages of the COVID-19 infection in Vero E6 cells [20-22].
As early as 2004, in vitro inhibition of severe acute respiratory syndrome coronavirus by Chloroquine has been demonstrated by Keyaerts et al, and during February 2020, Gao, Tian , and Yang reported a benefit of Chloroquine phosphate in SARS-CoV-2 associated pneumonia [23-24]. Figure 4 shows the chemical structure of Chloroquine. Table-4 summarizes the information relevant to the clinical use of Chloroquine.
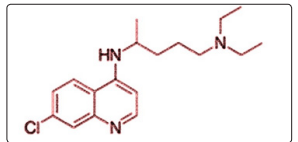
Figure 4: The Chemical Structure of Chloroquine
| Chloroquine was developed by Hans Andersag and Bayer laboratories in 1934, but was not used for a decade, because it was considered too toxic for human use. Thereafter, it was introduced into clinical the prophylaxis of malaria. |
| Approval and Value |
|---|
| Chloroquine is on the World Health Organization’s list of essential medicines which is the list of the medicines needed in a health system that are considered to be the safest and most effective. |
| Antiviral Activities |
| Chloroquine antiviral effects are attributed to increasing late Endosome or lysosomal pH, leading to impairment of the release of the virus from the endosome or lysosome, and thus preventing the release of virus genetic material into the cell and therefore preventing its replication. Chloroquine also inhibits or preventing viral RNA dependent RNA polymerase by acting as a zinc ionophore, allowing extra-cellular zinc to enter the cell. |
| Administration |
| Oral |
| Common and Minor Side Effects |
| Muscle problems Gastrointestinal Nausea, anorexia, vomiting, diarrhea, abdominal cramps Skin rash and easy bruising Pruritus Headache Legs and ankles swelling Unpleasant metallic taste |
| Possible Serious Side Effects |
| Blurred vision Muscle weakness Neutropenia, reversible agranulocytosis, and thrombocytopenia Pancytopenia and aplastic anemia, Cardiomyopathy Shortness of breath Hearing impairment and tinnitus Uncontrolled movements of tongue and face twitching |
| Possible Serious Side Effectss |
| Mental/mood changes including confusion, personality changes, unusual thoughts/behavior, depression, feeling being watched, hallucinating Flue-like illness with high fever, severe chills, and persistent sore throat |
| Use During Pregnancy |
| It is generally considered safe during pregnancy |
| Main Indications |
| 1-Treatment and prevention of malaria from Plasmodium vivax, P. ovale, and P. Malaria, but not malaria caused by Plasmodium falciparum. 2-Treatment of amoebic liver abscess resistant to metronidazole and other nitroimidazole within 5 days or intolerance to metronidazole or a nitroimidazole. 3-Treatment of autoimmune disorders, such as rheumatoid arthritis and lupus erythematosus. |
Baron et al (2020) emphasized that Teicoplanin was previously reported to be effective in inhibiting the first stage of MERScoronavirus viral cycle in human cells, was also active against the SARS-Cov-2. In addition, Wang et al (2016) Teicoplanin can inhibit Ebola pseudovirus infection by blocking virus entry in the low micromolar range [25-26]. It is also able to block the MERS and SARS envelope Pseudotyping viruses.
Teicoplanin is a semi-synthetic glycopeptide antibiotic used in the prophylaxis and treatment of serious infections caused by Grampositive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis. It acts by inhibiting bacterial cell wall synthesis, and its spectrum of activity similar to vancomycin. Oral Teicoplanin has been shown to be effective in the treatment of pseudomembranous colitis and Clostridium difficile-associated diarrhea, with comparable efficacy with vancomycin [1-3]. Figure 5 shows the chemical structure of Teicoplanin.
Teicoplanin can be used in the doses suggested by Sato et al (2006) [27]. A loading dose of 400 or 800 mg can be given on the first day, followed by maintenance dose of 400mg. In areas where serial tests are available for asymptomatic patients, a dose of 400mg can be given for to days with aim of achieving early negative test and clearance of the virus, and thus reducing its spread [1-4].
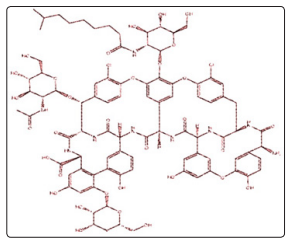
Figure 5: A Sketch of the Chemical Structure of Teicoplanin
Azithromycin, a macrolide antibiotic is effective against rhinovirus, respiratory syncytial virus, and influenza virus, and can also inhibit Zika and Ebola viruses. Tran et al. indicated that influenza progeny virus replication was remarkably inhibited by treating influenza virus with azithromycin before infection [28-31].
A study treated two groups of SARS-CoV-2 patients and compared the effect of treatment with sixteen SARS-CoV-2 control patients. Six patients (First group) were treated with Hydroxychloroquine (200 mg, 3 times daily, for 10 days) plus azithromycin (500 mg on first day, followed by 250 mg daily for the next 4 days), 14 patients were treated with Hydroxychloroquine as a single drug. On the sixth day of treatment, 100% of patients treated with Hydroxychloroquine plus azithromycin (First group) experience virological cure. Only 57.1% of the patients treated with Hydroxychloroquine as a single drug (Second group) and 12.5% in the control group experienced virological cure (P< 0.001). Thereafter, one patient, who was treated with Hydroxychloroquine as a single drug (in the second group) and continued to have PCR-positive at the sixth day of treatment, received azithromycin, and experience a virological cure. A recent SARS-CoV-2 infection ferret experiment suggested that Lopinavir-ritonavir, Hydroxychloroquine sulfate, and emtricitabine-tenofovir single drug treatments can marginally reduce clinical symptoms, but only emtricitabine-tenofovir treatment reduced virus titers in nasal. Nitazoxanide and ivermectin have recently been reported to have an activity against SARS-CoV-2 in vitro, but more evidence is required to recommend their clinical use. Favipiravir (T-705) is a broad-spectrum anti-RNA virus’s drug that was developed for the treatment of pandemic influenza virus infections. However, until this moment no published adequate evidence is available to support its use SARS-CoV-2 [32-35]. There is some preliminary evidence relying on data on homogenous coronaviruses and other pathogens suggesting that reducing the excessive inflammation, oxidation, and an exaggerated immune response which may contribute to SARS-CoV-2 pathological changes including a cytokine storm and progression to acute lung injury/acute respiratory distress syndrome and even death, may contribute to improving the outcome. In respiratory syncytial virus mice models, the use of melatonin was reported to cause down regulation of pro-inflammatory cytokine release, acute lung oxidative injury, and inflammatory cell activation. Melatonin, a safe, well-known anti-inflammatory and anti-oxidative molecule, is protective against severe respiratory symptoms caused by viruses and other pathogens. Melatonin was effective in critical care patients, probably acting by reducing vessel permeability, anxiety, sedation use, and improving sleeping quality. There is acceptable evidence suggesting that melatonin can limit virus-related diseases that enable its recommendation in the adjunctive supportive therapies [36-40].
SARS-CoV-2 has already defeated the efforts to prevent its spread and caused probably the most global pandemic in history. SARSCoV-2 continued to infect people and to take lives without the emergence of a treatment that is confirmed to have clinically a significant effectiveness in clinical treatment. Enormous spread could have possibly reduced by giving more attention to mild cases that could have played significant role in the relentless spread of the virus.The little available scientific evidence suggests that no single drug can result in a virological cure. However, Lopinavir/ ritonavir, Remdesivir, azithromycin, and Teicoplanin are possibly at the top of the list weapons of that have the potential to enable humans to win the fight against bat-human coronaviruses, but in condition that are used in suitable combinations. Chloroquine side effects include flue like febrile illness, shortness of breath and cardiomyopathy and therefore in our opinion it should be considered after Lopinavir/ritonavir, Remdesivir, and Teicoplanin. Lopinavir/ritonavir was considered at the top of the list because it is more available than Remdesivir in many countries of the world, and it has more known profile of toxicity and side effects.
The early use of safe well-known therapeutic agents having the potential to control the virus such as azithromycin, and Teicoplanin can help in preventing milder cases from spreading the virus, and also may prevent the progression to serious pneumonia and significant respiratory distress. However, effective treatment for more severe cases can also be achieved by the early use of drugcombinations that may include Lopinavir/ritonavir + ribavirin + azithromycin + Teicoplanin, Remdesivir + azithromycin, + Teicoplanin. The addition of low dose Chloroquine can also be considered in patients with healthy hearts.
Some of the sketches this paper was published before in our previous publication and the author have their copyright.
Conflict of interests: None.
