Author(s): Abdulbasit Andijany*, Rehab Fadag, Rasha Alsahafi, Bassem Bukhari and Walaa Felemban
Background: Many Anatomical Pathology laboratories do not have a barcoding system or an anatomical pathology laboratory information system that allow for better workflow by tracking of specimens and orders, quality control documentation, statistics calculation, search and data retrieval for research purposes.
Aim: This paper will show how Google sheet can be used as an alternative using the checkboxes, the electronic edit history and other features.
Materials and Methods: Google accounts were created then Google sheets were designed with specific details and the daily quality log was designed to show compliances to national and international standards.
Results: Descriptive templates allowed for tracking of specimens and pathologists’ requisitions, quality control documentation, statistics calculation, search and data retrieval for research purposes.
Conclusion: Google sheet can be used in the absence of barcoding systems or an anatomical pathology laboratory information system for tracking, documentation, search, research and monitoring of all activities by the supervisor as required by some accreditation body.
Numerous Laboratory Information Systems (LIS) are available; some are specific for Anatomical Pathology (APLIS) and allow for better workflow by tracking specimens using bar coding to specifically inform pathologists how far the specimens have progressed and where the specimens can be located within the lab whether that be the microtomy bench, staining machine or grossing station. APLIS also maximized efficiency by allowing pathologists to make additional requests (such as recuts, special stains and/ or immunohistochemistry), search for a specific diagnosis and retrieve data for research purposes [1-3]. Unfortunately, for some AP Laboratories, barcoding and/or an APLIS is not available and their LIS is part of the Hospital Information System (HIS) that does not tailor to the needs of Anatomical Pathology. Consequently, paper requests are used and pathologists are therefore unable to track their specimens or their additional requests. Another problem with paper requests is that they could be misplaced or lost leading to wasted time and manpower, errors are more prone to occur for example due to misreading numbers; the number 4 could be read as 9 and as a result, the turnaround time (TAT) is affected.
Google sheets allow for i) the creating and editing of data by multiple users at the same time from their phones, computers or tablets, ii) chatting, commenting and tagging of other users, iii) data retrieving by the “search” function for a specific disease or for research purposes, iv) real time tracking of data (who wrote, who deleted or who replaced the data) with the ability to restore previous versions and v) real time tracking of orders from their start time, status, completion and quality control by the “checkboxes” function which also informs other users when they were ticked and by whom [4]. These features allow for documentation of activities, help improve communication between staff, and allows staff to carrying out of their duties both without paper and more efficiently especially with the absence of APLIS and/ or barcoding systems.
Patients’ privacy could be maintained while using Google sheets by using only specimen accessioning numbers and avoiding the use of patients' names, ages and medical record numbers, provided that the sheets are only shared with known users and not publicly. In this paper, we will show our way for specimen tracking, dealing with additional requests (recuts, Special stains & Immunohistochemistry), quality control and statistics using Google sheets and how they look after implementation. This paper was approved by the Research and Ethics Committee, reference ethical number: REC 412.
• Google accounts, if non-existents, were created by/for consultant pathologists, residents’ office (not their personal Gmail), AP lab (receiving/releasing area), AP supervisor office and cytology supervisor office.
• Google Sheets was used to create the following:
• Template for histology specimens that contains columns; for the date, accession numbers, source of the specimen, number of blocks, initial of the pathologist in-charge, check boxes for residents/consultants for completion of sign out, check boxes for the completion of the H&E or other special stains, number of slides and check boxes for quality checking, fault/s and corrective action/s.
• Template for the additional requests by consultants/residents that contains the date, “Tests/orders” column (e.g. recuts/ levels/deepers, SS and/or IHC), initials of the ordering pathologist, time of order, check boxes for “In-progress”, “Completed” and “Quality checking”, faults, corrective actions and number of total slides for statistical calculation.
• Template for daily QC that covers quality/feedback of the grossed specimen, quality of the routine tissue processor, quality of the microwave tissue processor, quality of autoembedding machine, quality of histologic preparations, quality of frozen sections, daily QC for H&E stain and the staining machine, daily QC for Pap stain and Thinprep machine, daily QC for Diff-Quick stain and names of the checking and reviewing persons with check boxes to indicate their approval.
• Template for cytology specimens that shows the date, specimen accession number and source of the specimen, number of slides, interpretation of the cytotechnologist and final interpretation, time in screening, QC, corrective actions and check box for the pathologist for the completion of the sign out.
• All templates were made available as hard copies in the case of possible system fall-down and/or unavailability of the internet.
• All sheets are saved electronically and could be printed and stored as hard copies
Histology and cytology sheets (figures 1&2) allow the pathologist to view and tick off completed cases. These sheets allow for easy visualization of daily cases and allow pathologist to keep track of pending cases. The histology ordering sheets (fig. 3) allows for real time ordering of levels, recuts, and ancillary tests as well as allowing the pathologist to follow the progress of his/her order and ensure that it has undergone QC check. The Daily Quality log (fig.4) ensures quality of each step of specimen processing.
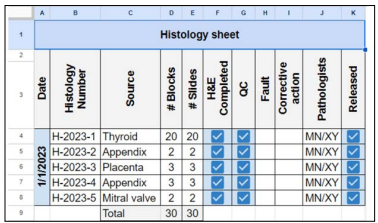
Figure 1: Histology Sheet Legends: H&E: Hematoxylin & Eosin Stain, QC: Quality Control
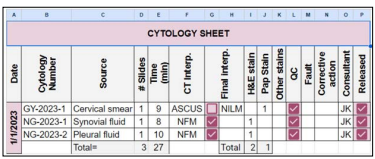
Figure 2: Cytology Sheet
Legends: ASCUS: Atypical Squamous Cell of Undetermined Significance, H&E: Hematoxylin & Eosin Stain, Interp.: Interpretation, NFM: Negative for Malignancy, PAP: Papanicolaou Stain, QC: Quality Control.
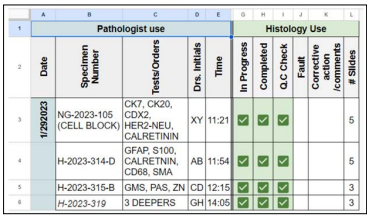
Figure 3: Ordering Sheet
Legends: QC: Quality Control.
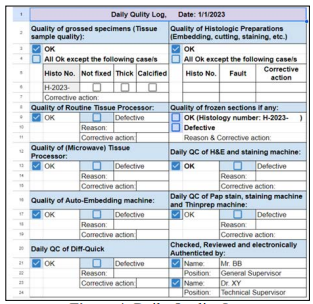
Figure 4: Daily Quality Log
A typical histology sheet (Figure 1:) shows that all check-boxes are checked for completion of the H&E stain, QC and completion of the sign out of all cases on that day by the consultant pathologist which, therefore, assures that no forgotten cases or pending for approval (after typing of the report by secretaries) are left behind. Figure 2: shows the elements that the College of American Pathologist (CAP) requires from cytotechnologists (CT) which are documenting the total number of slides in a 24-hour, time in screening and interpretation results for each case, CAP also require that cytological statistics for gynecological specimens are maintained and compared to benchmarking data [5], such requirements can be implemented in the cytology sheet but we use a separate sheet for cytological statistics. For competency assessment, CT interpretation versus the final report can be used from the sheet. Additional requests (recuts, SS and/or IHC) are documented and tracked (Figure 3:), instead of the pathologists coming into the lab and writing down their orders in a file or handing in a piece of paper that can get lost. Pathologists can now order from their PC, tablets or even their smart phones from their offices or homes and are able to know whether their requests are completed and ready for examination. The total number of slides in this sheet are calculated on a monthly basis. Figure 4: shows all the requirements by the CAP and The Saudi Central Board for Accreditation of Healthcare Institutes (CBAHI) for Anatomic pathology and Cytopathology that must be documented [5-7]. The technical supervisor and the sign-out consultant pathologist with the help of these sheets and the previously mentioned sheets can have a comprehensive overview of all practices and follow up where necessary
In the absence of barcoding and/or Anatomical Pathology Laboratory Information System, Google Sheet can be used to track specimens and additional requests, manage quality control and retrieve data for statistics, search and research in Anatomical Pathology and Cytopathology Laboratories. It can also help the technical supervisor to continuously monitor the laboratory activities and take appropriate actions when needed.
