Author(s): Anubha Bajaj
Desmoplastic fibroblastoma is a distinctive, exceptional, benign, fibrous soft tissue tumefaction of mesenchymal origin confined to the subcutaneous tissue. Initially scripted by Evans in 1995, the neoplasm is additionally nomenclated as “collagenous fibroma” [1]. The predominantly fibroblastic neoplasm is minimally cellular and is composed of reactive fibroblasts intermixed with abundant collagen fibres. The neoplasm originating from connective tissue is instilled with a potential for localized aggressiveness although distant metastasis is undocumented.
The neoplasm is discerned in adults with a peak incidence between fifth decade to seventh decade and a median age of disease discernment at 50 years. An estimated 70% neoplasms are delineated in adults. A male preponderance is observed. Tumour magnitude varies from 1 centimetre to 20 centimetres with a median diameter of 3.0 centimetres although majority of neoplasms are below < 4 centimetres when surgically excised [2]
Generally the neoplasm is exhibited within upper and lower extremity although a wide anatomic distribution of lesions is exemplified such as posterior neck, arm, shoulder, foot, upper dorsal region, abdominal wall, gluteal region, axilla, tongue, lacrimal gland, parotid gland, jaw, metaphysis of long bones or palate. Desmoplastic fibroblastoma of the bone can be contemplated as an intraosseous counterpart of soft tissue fibromatosis [2, 3].
Certain instances demonstrate a genetic translocation of chromosome 11q12. An estimated 57% tumefaction are present for a duration exceeding > 6 months and around 32% tumours manifest beyond > a year. Around 70% of desmoplastic fibroblastomas are confined to the subcutaneous tissue whereas around 25% tumours expand into adjacent skeletal muscle as the neoplasm is contemplated to be imbued with an invasive potential. Majority of neoplasms are accompanied by microscopic extension into abutting subcutaneous adipose tissue [2, 3].
Majority of desmoplastic fibroblastomas are asymptomatic. Notwithstanding, desmoplastic fibroblastoma represents as a prominent, firm, well circumscribed, spherical or elliptical, painless, gradually progressive tumefaction of long standing duration, commonly situated within subcutaneous tissue or skeletal muscle. Tumour nodule can be hard, tender, palpable, and inadequately mobile and generates an asymmetry of incriminated organ [2, 3].
Invasive desmoplastic fibroblastoma denominates pertinent manifestations such as brief clinical duration, aggressive features, preferential anatomical site, enlarged tumefaction and pain. Although bone infiltration is exceptional, the tumefaction can arise within skeletal muscle and infiltrate abutting bone, thus engendering pain and restricted range of joint motion. Histologically Elucidation Macroscopically, an elongated, well circumscribed, lobulated, elliptical or disc shaped, firm nodule is delineated. Usually, tumour diameter varies between one centimetre to 4 centimetres. The well circumscribed tumefaction may be lobulated. Cut surface is homogenous, firm, solid, greyish/ white and simulates cartilage [3, 4].
On microscopy, a relatively hypo-cellular tumefaction discerned which is composed of proliferation of bland, spindle-shaped or stellate cells enmeshed within a dense, fibrotic, collagenous or fibromyxoid stroma demonstrating significant quantities of stromal mucin and minimal vascularity. Alcian blue stain highlights the stromal mucin. Mitotic figures are exceptional or absent [4, 5].
The uniform, variably cellular neoplasm delineates widely spaced, bland, spindle-shaped to stellate cells embedded within a myxo-collagenous stroma. Mature fibrous connective tissue is interspersed with spindle- shaped fibroblasts along with an abundance of collagen bundles. The pauci- cellular neoplasm is composed of bland, spindle-shaped or stellate cells admixed with reactive fibroblasts and myo-fibroblasts.
The cellular component is separated by abundant quantities of collagen and a variable intermingling of myxoid stroma. Fibroblasts demonstrate an amphophilic cytoplasm, vesicular nuclei and distinct nucleoli [4, 5]. Tumour parenchyma is composed of proliferating spindle-shaped cells with ill-defined cellular margin. A patternless configuration of proliferating, bland, spindle-shaped cells is enunciated, disseminated within a fibrotic to myxoid matrix. Besides, tumour cells may configure a fascicular or swirling pattern with an encompassing non- collagenized stroma [3, 5].
The non- encapsulated tumour is constituted by spindle-shaped cells configuring abridged fascicles. Tumour parenchyma is enveloped within a collagenous stroma. Tumour cells are incorporated with elliptical to elongated nuclei with an absence of polymorphism, cellular atypia or mitotic activity. Nuclear atypia or tumour necrosis is absent. Focal tumour infiltration is frequently discerned within circumscribing skeletal muscle and adipose tissue. On ultrastructural examination, cells of fibroblastic or myo-fibroblastic lineage are denominated. Tumour cells can depict fibronexus junctions which are indicative of myofibroblastic differentiation. The neoplasm denominates pertinent genomic anomalies such as chromosomal translocation t (2; 11) (q31; q12) or anomalies of chromosome 11q12 [4, 5].
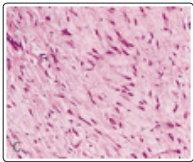
Figure 1:o Desmoplastic fibroblastoma delineating spindle-shaped and stellate cells intermixed within a dense, fibrotic, minimally vascular stroma with abundant mucin [9].
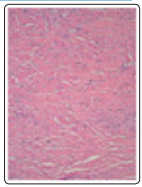
Figure 2: Desmoplastic fibroblastic depicting spindle-shaped and stellate cells intermingled within an intensely fibrotic stroma with few blood vessels and abundant stromal mucin [10].
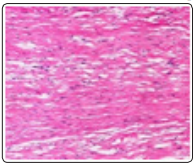
Figure 3: Desmoplastic fibroblastoma demonstrating predominant spindle-shaped cells commingled within an intense, fibrotic stroma with minimal vascular articulations and extensive mucin [11].
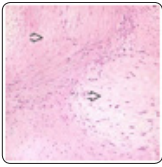
Figure 4: Desmoplastic fibroblastoma exhibiting spindle-shaped and stellate cells admixed with dense, fibrotic stroma, few blood vessels and abundant stromal mucin [12].
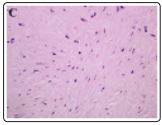
Figure 5: Desmoplastic fibroblastoma demonstrating spindleshaped and stellate cells commixed within a collagenous stroma with foci of stromal mucin [13].
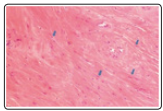
Figure 6: Desmoplastic fibroblastoma depicting disseminated uniform, spindle-shaped cells interspersed within collagenous stroma with extensive mucin [14].
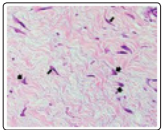
Figure 7: Desmoplastic fibroblastoma enunciating uniform, spindle-shaped cells commingled with collagenous stroma and mucin deposits within the stroma [15].
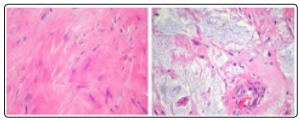
Figure 8: Desmoplastic fibroblastoma delineating clusters of bland, spindle-shaped cells intermingled with collagenous stroma with foci of stromal mucin and lack of atypia [16].
Desmoplastic fibroblastoma is diffusely immune reactive to vimentin. Additionally, tumour cells are diffusely immune reactive to FOS-related antigen1 (FOSL1). Vascular articulations are immune reactive to CD31 and smooth muscle actin (SMA). Also, specific instances can demonstrate focal immune reactivity to smooth muscle actin (SMA). Certain tumour cells are immune reactive to CD34. Tumour cell nuclei can occasionally depict an intense, diffuse immune reactivity to Β- catenin. The tumefaction comprehensively (100%) delineates a Ki-67 labelling index of below<5%, thereby indicating a minimal tumour proliferation. The neoplasm is immune non reactive to α-smooth muscle actin (α-SMA), muscle specific actin (MSA), epithelial membrane antigen (EMA), CD34, S100 protein, keratin, desmin or Β- catenin [2, 3].
Desmoplastic fibroblastoma requires a segregation from diverse, fibrous, soft tissue tumours such as
On plain radiography, a well circumscribed, elliptical lesion is observed. An osteolytic lesion with sclerotic margins is denominated within tumefaction abutting bony surfaces [2, 3]. Bone lesions can be unilocular or multilocular with well-defined or poorly defined borders or delineate an irregular, radiolucent outline. Upon imaging, the neoplasm can depict a lobulated, soapbubble like appearance, thereby mimicking adjunctive lesions such as ameloblastoma, myxoma, aneurysmal bone cyst, central haemangioma or eosinophilic granuloma. However, emergence of course, irregular fibrous tissue septa are indicative of desmoplastic fibroblastoma [3, 4].
Contrast- enhanced computerized tomography (CT) demonstrates an osteolytic soft tissue mass with infiltration into adjacent bone. Alternatively, a radiolucent, well defined, expansible lesion is denominated [3, 4]. On magnetic resonance imaging (MRI), a tumefaction with uninvolved tumour perimeter is delineated. The lesion depicts minimal intensity upon T1 and T2 weighted imaging with reduced T1 inversion recovery, indicative of occurrence of collagen- predominant fibrous tumours.
Adoption of gadolinium contrast medium depicts an enhancement of neoplastic peripheral rim which distinguishes desmoplastic fibroblastoma from adjunctive fibrous tumours. MRI can also evaluate precise proportion of soft tissue infiltration of the neoplasm. Clinical and radiographic features are often indicative of desmoplastic fibroblastoma although cogent histological examination is imperative for definitive tumour discernment [3, 4].
Generally, conservative management with surgical extermination of neoplasm is satisfactory as the tumefaction is devoid of localized reoccurrence or distant metastasis. Comprehensive surgical eradication of the lesion is an optimal treatment strategy. The tumefaction is adequately treated with simple or marginal surgical extermination with eradication of a tumour-free perimeter of normal tissue [8].
Intra-oral desmoplastic fibroblastoma can be extracted with enucleation and curettage while ensuring a tumour-free perimeter of 1centimeter. Radiotherapy can be alternatively employed to treat inoperable neoplasms [7, 8]. Prognostic outcomes of desmoplastic fibroblastoma are favourable despite the presence of an invasive potential. Localized tumour reoccurrence is absent [7, 8].
