The Role of Cytokines and Cellular Receptors in the Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
Author(s): <p>Usha Kellampalli, Hesham Mohei and Irina Vlasova-St. Louis</p>
Abstract
Tuberculosis (TB) is the most prevalent opportunistic infection in patients with acquired immunodeficiency syndrome (AIDS) and the major reason for morbidity and mortality. In this review, we focus on pathological immune restoration in AIDS patients after antiretroviral therapies (ART) initiation. This manuscript discusses the importance of T cell immunity for antigen-specific immune reconstitution and the production of cytokines, which are divided into 2 subgroups: interferons (IFN), and interleukins (IL). We overview the role they play in connection between innate and adaptive immunity during immune restoration. We describe how hypercytokinemia produced by innate and adaptive immune cells, leads to the manifestations of tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) in AIDS patients during the recovery of CD4+ T cells on ART. We conclude with a brief synopsis of treatment approaches.
Immune Reconstitution Inflammatory Syndrome in AIDS Patients
Tuberculosis (TB) is the most prevalent opportunistic infection in latestage HIV-infected individuals and the major reason for morbidity and mortality [1]. Furthermore, the risk of TB- associated IRIS remains a major clinical concern, after patients initiate antiretroviral therapy [2]. The foremost common clinical features of TB-IRIS are fever and worsening respiratory symptoms with pulmonary infiltrates, mediastinal lymphadenopathy, and pleural effusions.
Extrapulmonary TB-IRIS manifestations include acute nephropathy, intracranial tuberculomas, tuberculous meningitis, skin or visceral abscesses, osteomyelitis, epididymo-orchitis [3,4]. Rates of morbidity and mortality attributable to paradoxical tuberculosis associated IRIS is higher in resource limited settings where diagnostic and treatment options are restricted [5-8]. The manifestations of TB-IRIS occur within 3 months of ART initiation, re-initiation, or regimen change because of treatment failure. The British HIV Association has issued guidelines for starting the TB treatment with ART according to CD4+ cell count. If the CD4+ cell count is greater than 200 cells/µl, ART is started 6 months after completing TB treatment. If CD4+ cells are in the range of 100-200; ART is started 2 months after starting TB treatment. If CD4+ cell count is less than 100, both anti-tuberculosis drugs and ART can be started together [9].
Kinetics of Innate and Adaptive Immune Reconstitution in TB-IRIS
Kinetics of cytokine expression and secretion in blood and cerebrospinal fluid (CSF) during immune reconstitution in TB-AIDS patients have been described [10-12]. Peripheral blood mononuclear cells from patients co-infected with HIV and Mycobacterium tuberculosis (MTB) demonstrated increased blood levels of the innate pro-inflammatory cytokines IL1, IL6, IL8, and TNFA (tumor necrosis factor alfa) in vitro after stimulation with heat-killed MTB [13,14]. Patients with TB-IRIS have shown increased levels of IL6 and IL18 and low levels of IL27 before ART commencement, followed by the expansion of inflammatory monocyte subsets and inflammasome activation during IRIS events [15-19].
Numerous cytokines increase in plasma and serum after ART initiation, such as CRP (C- reactive protein), granulocyte-colony stimulating factor (GCSF), interleukins IL1B, IL1RA, IL6, IL8, IL18, TNFA and soluble tissue factor [20]. The foremost consistently elevated cytokines during TB-IRIS events are IL6, TNFA, and IFNG (interferon gamma) [21].
Abnormal frequencies of chemokine receptor expression on CD4+ T cells is associated with TB-IRIS. For example, an increase in CXCR3+CCR6-CD4+ T cells and a decrease in CXCR3- CCR6+CD4+ Th17 lymphocytes have been reported in TB-IRIS cases from pre-ART to 6 weeks of post-ART initiation [22]. Along with these cytokines, the pre-ART increases in plasma concentrations of IL10, monocyte chemoattractant protein 1 (MCP1/CCL2), and eotaxin render TB patients susceptible to TBIRIS and early mortality. Thus, dyscytokinemia during immune reconstitution created by innate and adaptive immune cells is behind IRIS symptoms [14,16].
Recovery of T Helper Cells and TB-IRIS Events
Several studies assessed baseline and CD4+ T cell count kinetics in patients who developed IRIS and compared with those who died from other causes after ART initiation [23]. The recovery of CD4+ T cells was similar in patients who developed TB-IRIS and survived, as compared to patients with uneventful recovery on ART. In the early stages of immune restoration, CD4+ T cell memory dominated phenotype is observed in TB-IRIS patients, which confers protection against mycobacterium tuberculosis reinfection/ reactivation or relapse [24-26]. IRIS patients reconstitute a higher proportion of helper CD4+ T cells during 6 months post-ART as compared to non-IRIS patients [27]. These CD4+ T cells (together with CD8+ T cells) secrete higher IFNG in response to TB antigens and many other pro-inflammatory cytokines which may lead to cytokine storm [28,29]. The assessment of CCR5 and CXCR3 expressions on T cells to investigate the role of Th1 responses in TB-IRIS, before and after ART showed that TB-IRIS patients had higher proportions of CCR5+CD4+ T cells at week 6, which also associated with high viral loads. There was also an increase in the proportion of CD4+ T cells co-expressing CCR5+ and CXCR3+ in the TB-IRIS group compared with the non-TB-IRIS group [30,31].
Cells of the Innate Immune System in IRIS
Innate immune cell types, including natural killer (NK) cells and myeloid cells, are also linked to TB-IRIS development [25,32,33]. There are major differences between the kinetics of favorable immune reconstitution and that which is accompanied with IRIS. Activation of cytotoxic NK and T cells in peripheral blood mononuclear cell population (PBMCs) from IRIS patients resulted in a higher expression of cytotoxic mediators (perforin and granzyme B) [34]. Higher NK cell degranulation capacity in IRIS patients before ART and after 2 weeks of TB treatment initiation has been reported in IRIS patients, followed by decreased expression of NK cell activating- receptors (NKp30, NKp46, NKG2D (NK group 2D)) [33]. NK cell activity is controlled by cytokines (mainly IL12, IL15, IL18, and IFNA) and by a complex repertoire of activatory and inhibitory receptors. It has been shown that NK/T cells have a unique phenotype of effector T cell receptor subunits expression, which enhances effector functionality with higher degranulation potential [35,36]. The expression of killer cell immunoglobulin-like receptors (CD158a) was higher in TB-IRIS than non-IRIS patients before ART, but the expression of NKG2D (CD314+) on NK/T cells was lower in HIV-TB patients after ART initiation [37].
The activation of the inflammasome by monocytes and macrophages may play a role in systemic inflammation via the production of nitric oxide (NO), IL1 and IL18 in patients who develop TB-IRIS after commencing ART [38-40]. Moreover, live mycobacterium tuberculosis and MTB antigens are known to activate (NOD-, LRRand pyrin domain-containing protein 3) NLRP3 and inflammasome cascade, causing significant damage to target cells and tissues [40- 42]. Thus, it is not surprising that immune activation occurs via NLR-inflammasome pathways, representing exaggerated innate cells’ response toward ongoing viral replication and microbial antigens. It had been recently shown that the inflammasome pathway drives CD4+ T cell depletion in HIV infection and delayed immune reconstitution. In TB-IRIS patients with symptoms of deterioration of the central nervous system, the increased inflammasome activation may represent a peripheral biomarker of brain inflammation that crosses the blood-brain barrier [16,17,43-45]. These research reports discussed the inflammasome pathway as a driver of CD4+ T cell depletion in HIV1 infection that delayed immune reconstitution and played a critical role in IRIS pathogenesis [46].
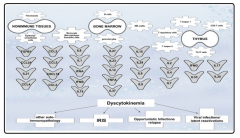
Figure 1: Dyscytokinaemia that leads to or accompanies the tuberculosis-associated immune reconstitution disorders.
Treatment Approaches to Combat IRIS
Since hypercytokinemia discussed above is a major driver of IRIS symptoms, corticosteroid therapies have demonstrated improved outcomes due to increased transcription of anti-inflammatory mediators and decreased transcription of proinflammatory cytokines and chemokines [47]. However, corticosteroids have been shown to decrease T-cell survival by enhancing apoptosis [48]. The use of prednisone to treat TB-IRIS, cut down acute symptoms in the short term [49,50]. The addition of short duration of IFNG or IFNA to prednisone treatment was useful to bring back impaired communication between innate and adaptive immune branches [51-54]. The serum concentration of cytokines such as IL10, IL12p40, IFNG, and chemokine CXCL10 decreased during 4 weeks of prednisone therapy, but increased in the placebo group, further emphasizing a pathological role for hypercytokinemia in TB-IRIS (figure 1) [47].
Neutralization of IL6 with a monoclonal antibody decreased disease pathology and extended survival in preclinical models, yet to be confirmed in phase 1 studies in humans [21]. Anti-TNF agents such as antibodies, chloroquine or thalidomide can be useful if administered with ART to prevent or treat TB-meningitis and TB-IRIS [54,55]. Other drugs such as pentoxifylline and hydroxychloroquine had also been used to treat IRIS patients, with some reported benefits [56- 58]. Leukotriene antagonist, Montelukast, has shown successful outcomes in treating steroid-refractory IRIS patients [59,60]. Leukotrienes trigger broad antimicrobial, proinflammatory effects due to leukocyte (neutrophil particularly) recruitment to sites of inflammation, and amplification systemic immune responses [61,62]. Montelukast reduces leukotriene-driven inflammatory response without creating significant immunosuppression. Formal clinical trials need to be conducted to define the success of these therapies, and assess the effectiveness and the duration of treatment required. Since M. tuberculosis infection increases CCR5+ expression on T cells and CCR5+CD4+ T cells accumulate in the lung during TBIRIS, a CCR5 inhibitor therapy could be considered as a therapeutic strategy to prevent pulmonary TB-IRIS in HIV- infected patients when given ART.
Conclusion and future prospective
Dyscytokinemia (figure 1) plays a major role in the pathogenesis of immune reconstitution disorders [63]. Cytokines, that are produced by T, NK, NK/T cells, and innate immune cells play a profound role in managing the immune responses. As discussed above, abnormal frequencies of chemokine receptor expression, and high levels of IL6, IL10, TNFA, and IFNG represent the signature of TB-IRIS. Since hypercytokinemia is a major driver of IRIS pathogenesis, immunotherapies have shown to improved patients’ symptoms.
Conflict of Interests
The authors declare no conflict of interest.
Funding
This project was part of U01AI089244
References
- Trk ME (2015) Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull 113:
- Chang CC, Sheikh V, Sereti I, M A French (2014) Immune reconstitution disorders in patients with HIV infection: from pathogenesis to prevention and treatment. Curr HIV/AIDS Rep 11:
- Manosuthi W, Van Tieu H, Mankatitham W, Aroon Lueangniyomkul, Jintanat Ananworanich, et al. (2009) Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 23:
- Bosamiya SS (2011) the immune reconstitution inflammatory syndrome. Indian J Dermatol 56:
- Burman W, Weis S, Vernon A, A Khan, D Benator, et (20047) Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis 11: 1282-
- Lawn SD, Myer L, Bekker LG, Robin Wood (2007) Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS 21:
- Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph (2006) Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect 53:
- Lawn SD, Bekker LG, Miller RF (2005) Immune reconstitution disease associated with mycobacterial infections in HIVinfected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5:
- Pozniak AL, Miller RF, Lipman MC, A R Freedman, L P Ormerod, et al. (2005) BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med 6:
- Bloom CI, Graham CM, Berry MP, Wilkinson KA, Oni T, et al. (2012) Detectable changes in the blood transcriptome are present after two weeks of antituberculosis therapy. PLoS 7:
- Berry MP, Graham CM, McNab FW, Xu Z, Bloch SAA, et al. (2010) An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:
- Musselwhite LW, Andrade BB, Ellenberg SS, Tierney A, Zamudio, et al. (2016) Vitamin D, D-dimer, Interferon gamma, and sCD14 Levels are Independently Associated with Immune Reconstitution Inflammatory Syndrome: A Prospective, International Study. EBioMedicine 4:
- Tan DB, Lim A, Yong YK, Ponnampalavanar S, Sharifah Omar, et al. (2011) TLR2-induced cytokine responses may characterize HIV-infected patients experiencing mycobacterial immune restoration disease. Aids 25:
- Tadokera R, Meintjes G, Skolimowska KH, Wilkinson, Matthews, et al.(2011) Hypercytokinaemia accompanies HIVtuberculosis immune reconstitution inflammatory Eur Respir J 37:
- Narendran G, Andrade BB, Porter BO, Chandrasekhar C, Venkatesan P, et al. (2013) Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 8:
- Oliver BG, Elliott JH, Price P, Phillips M, Saphonn V, et (2010) Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis 202:
- Tan HY, Yong YK, Andrade BB, Shankar EM, Ponnampalavanar S, et al. (205) Plasma interleukin-18 levels are a biomarker of innate immune responses that predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 29:
- Tan HY, Yong YK, Shankar EM, Paukovics G, Ellegrd R, et al. (2016) Aberrant Inflammasome Activation Characterizes Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. J Immunol 196:
- Andrade BB, Singh A, Narendran G, Schechter ME, Nayak K, et al. (2014) Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosisassociated immune reconstitution inflammatory PLoS Pathog 10:
- Grant PM, Komarow L, Lederman MM, Pahwa S, Zolopa AR, et al. (2012) Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. J Infect Dis 206:
- Barber DL, Andrade BB, McBerry C, Irini S, Alan S, et (2014) Role of IL-6 in Mycobacterium avium-- associated immune reconstitution inflammatory syndrome. J Immunol 192: 676-
- Silveira-Mattos PS, Narendran G, Akrami K, Fukutani KF, Anbalagan S, et al. (2019) Author Correction: Differential expression of CXCR3 and CCR6 on CD4(+) T-lymphocytes with distinct memory phenotypes characterizes tuberculosisassociated immune reconstitution inflammatory In: Sci Rep. (ed)^(eds): England
- Ravimohan S, Tamuhla N, Steenhoff AP, LetlhogileR, Nfanyana K, et al. (2015) Immunological profiling of tuberculosis- associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis 15:
- Ma J, Zhao F, Su W, Li Q, Li J, et al. (2015) Zinc finger and interferon-stimulated genes play a vital role in TB-IRIS following HAART in AIDS. Per Med 15:
- Haridas V, Pean P, Jasenosky LD, Madec Y, Laureillard D, et al.(2015) TB-IRIS, T-cell activation, and remodeling of the T-cell compartment in highly immunosuppressed HIVinfected patients with TB. AIDS 29:
- Verhofstede C, Nijhuis M, Vandekerckhove L (2012) Correlation of coreceptor usage and disease Curr Opin HIV AIDS 7:
- Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, et al. (2010) Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 116:
- Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, Veen KV, et al. (2008) Type 1 helper T cells and FoxP3- positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 178:
- Ruhwald M, Ravn P (2007) Immune reconstitution syndrome in tuberculosis and HIV-co- infected patients: Th1 explosion or cytokine storm? AIDS 21:
- Vignesh R, Kumarasamy N, Lim A, Solomon S, Murugavel KG, et al. (2013) TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr 64:
- Syrbe U, Siveke J, Hamann A (1999) Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol 21:
- Bourgarit A, Carcelain G, Samri A, Parizot C, Lafaurie M, et al. (2009) Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculinspecific CD4 Th1 cells and KIR-negative gammadelta T J Immunol 183:
- Pean P, Nerrienet E, Madec Y, Borand L, Laureillard D, et (2012) Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood 119:
- Wilkinson KA, Walker NF, Meintjes G, Deffur A, Nicol MP, et al. (2015) Cytotoxic mediators in paradoxical HIVtuberculosis immune reconstitution inflammatory J Immunol 194: 1748-
- Johansson SE, Rollman E, Chung AW, Center RJ, Hejdeman BO, et al. (2011) NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller Viral Immunol 24: 359-
- Walker NF, Opondo C, Meintjes G, Jhilmeet N, Friedland JS, et al. (2019) Invariant Natural Killer T cell dynamics in HIV-associated tuberculosis. Clin Infect Dis
- Pean P, Nouhin J, Ratana M, Madec Y, Borand L, et al. (2019) High Activation of T Cells and the 2. Front Immunol 10:
- Diedrich CR, Flynn JL (2011) HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun 79:
- Tomlinson GS, Bell LC, Walker NF, Tsang J, Brown JS, et al. (2014) HIV-1 infection of macrophages dysregulates innate immune responses to Mycobacterium tuberculosis by inhibition of interleukin-10. J Infect Dis 209:
- Dorhoi A, Nouailles G, Jrg S, Hagens K, Heinemann E, et al. (2012) Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol 42:
- Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, et (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:
- Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, et al. (2013) Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1b. Nat Immunol 14:
- Marais S, Lai RP, Wilkinson KA, et al. Inflammasome activation underlies central nervous system deterioration in HIV-associated tuberculosis. J Infect Dis
- Tran HT, Van den Bergh R, Loembe MM, Worodria W, Kizza HM, et al. (2013) Modulation of the complement system in monocytes contributes to tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS (London, England) 27:
- Tran HT, Van den Bergh R, Vu TN, Laukens K, Worodria W, et al. (2014) The role of monocytes in the development of Tuberculosis-associated Immune Reconstitution Inflammatory Syndrome. Immunobiology 219:
- Tan HY, Yong YK, Shankar EM, Paukovics G, Ellegrd R, et al. (2016) Aberrant Inflammasome Activation Characterizes Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. J Immunol 196:
- Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Tadokera R, et al.(2012) Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 186:
- Payne DN, Adcock IM (2001) Molecular mechanisms of corticosteroid actions. Paediatr Respir Rev 2:
- Meintjes G, Stek C, Blumenthal L, Thienemann F1, Schutz C, et al. (2018) Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N Engl J Med 379:
- Stek C, Schutz C, Blumenthal L, Thienemann F, Buyze J, et al. (2016) Preventing Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome in High-Risk Patients: Protocol of a Randomized Placebo-Controlled Trial of Prednisone (PredART Trial). JMIR Res Protoc 5:
- Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, et al. (2004) Recombinant interferon- gamma 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis 189:
- Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, et al. (2012) Adjunctive interferon-y immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26:
- Bosinger SE, Utay NS (2015) Type I interferon: understanding its role in HIV pathogenesis and therapy. Curr HIV/AIDS Rep 12:
- Wallis RS, van Vuuren C, Potgieter S (2009) Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis 48:
- Lai RP, Meintjes G, Wilkinson RJ (2016) HIV-1 tuberculosisassociated immune reconstitution inflammatory Semin Immunopathol 38:
- Bell HC, Heath CH, French MA (2005) Pulmonary Mycobacterium celatum immune restoration disease: immunopathology and response to corticosteroid AIDS 19: 2047-
- Wallis RS, Johnson JL, Okwera A, Nsubuga P, Whalen CC, et al. (1998) Pentoxifylline in human immunodeficiency viruspositive tuberculosis: safety at 4 years. J Infect Dis 178:
- John M, French MA (1998) Exacerbation of the inflammatory response to Mycobacterium tuberculosis after antiretroviral therapy. Med J Aust 169:
- Hardwick C, White D, Morris E, R A Breen, M Lipman (2006) Montelukast in the treatment of HIV associated immune reconstitution disease. Sex Transm Infect 2006; 82:
- Lipman MC, Carding SK (2007) Successful drug treatment of immune reconstitution disease with the leukotriene receptor antagonist, montelukast: a clue to pathogenesis? AIDS 21:
- Coffey MJ, Phare SM, George S, Golden MP, Kazanjian PH (1998) Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest 102:
- Peters-Golden M, Canetti C, Mancuso P, Coffey MJ (2005) Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 174:
- Mohei H, Kellampalli U, Vlasova-St.L I (2019) Immune Reconstitution Disorders: Spotlight on International journal of biomedical investigation 2: 1-21.
View PDF

