Author(s): <p>Ebrahim Salehifar and Homa Talabaki*</p>
ABSTRACT
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a condition related to cancer treatment. Although various drugs and protocols are recommended for prophylactics and treatment, no strategies have yet been proven effective completely. Several drug treatment regimens have been proven for neuropathy, which includes calcium channel blockers, opioids, anti-depressants, and anticonvulsants, but they are only partially effective and have adverse effects associated with their administrations that diminish the quality of life and an economic burden is imposed on the patient. On the one hand, to treat peripheral neuropathy, a single therapeutic agent is not sufficient. On the other hand, Plants are the source of a vast number of bioactive phytochemicals which can potentially treat disease and related complications together.
Objectives: Many clinical trials and animal experiments have assessed the potential role of herbal products in the treatment of neuropathy. Ziziphus jujube is one of the medicinal herbs that is generally safe and not toxic to humans. Many studies of the Ziziphus species have shown their therapeutic properties. The current article exhaustively reviews the phytochemical profiles of Ziziphus jujube in neuropathy.
Methods: Our purpose was to find all English published reports of anti-inflammatory and neuroprotective effect of ziziphus jujube. Biomedical databases comprised Web of Science PubMed, Scopus, and Google Scholar. We used “neuropathy, anti-inflammatory, neuroprotective, ziziphus jujube” as key research words.
Results: The present review article also highlights the most promising experimental data on Ziziphus extracts and pure active compounds in clinical trials and animal models of neuropathy.
Conclusion: This review would be a valuable resource for contemporary researchers in the field to understand the promising role of the Ziziphus jujube in neuropathy.
Chemotherapy-induced peripheral neuropathy (CIPN) is known due to the administration of several chemotherapy drugs, including platinum compounds, antitubulins, proteasome inhibitors, and immunomodulatory agents. This persecutory complication leads to dose reduction or discontinuation of chemotherapy regimens; also, it can negatively diminish the quality of life (QOL) of cancer survivors and affect functional activity, since up to 40% of these individuals even after the end of the therapeutic course may keep experiencing symptoms, such as pain and other types of disability, leading to significant challenges on their personal life. It is expressed in both sensory and motor symptoms. Sensory axonal neuropathy clinically manifests as numbness and parenthesis. Motor symptoms, usually manifest as a distal weakness such as a foot drop, and autonomic manifestations, including orthostatic hypotension, cardiovascular, erectile, or gastrointestinal disturbances [1]. Currently, several drug treatment regimens have been proven for neuropathy, which includes calcium channel blockers, opioids, anti-depressants, and anticonvulsants, but they are only partially effective and have adverse.
effects associated with their administrations that diminish the quality of life and an economic burden is imposed on the patient [2]. Medical herbs, as a paradigm of proactive medicine, have fewer side effects than chemical drugs, and patients have used or expressed interest in their usage for prophylactics and/or to improve the quality of life; additionally, such medicines have attracted the attention of scientists [3]. Many studies have pointed out that bioactive components derived from jujube fruit have significant nutritional and potential biological effects [4]. First in this study, we have a look on hypothesis for mechanism of platinum-induced peripheral neurotoxicity. In following, we review therapeutic properties of Z. jujube; then experimental works on animals and human with Z. jujube is covered and finally herbal formulas containing Z. jujube are presented.
In the current review article, the information was collected from a literature search using various computerizes databases as PubMed, Google Scholar, Scopus Library. Keywords such as Ziziphus jujube and Neuropathy were used to search literature from 2010 to 2022. All the preclinical and clinical studies mentioned in this review solely focus on anti-inflammatory and neuroprotective effect of ziziphus jujube especially in CIPN.
Mechanism of Action of Chemotherapy Agents in CIPN Neurotoxic anticancer drugs affect the peripheral sensory nerve by directly targeting the mitochondria and producing oxidative stress, functionally impairing ion channels, triggering immunological mechanisms through activation of satellite glial cells, and/or disruption of microtubules [1-4]. It is important to note that these various neurotoxic events are not necessarily related to the anticancer mechanisms of action for these agents and may account for the lack of effective treatment. It is hypothesized that a polytherapy which targets multiple mechanisms will most likely be the means to achieve neuroprotection. Different chemotherapies affect distinct components of the peripheral nervous system, from the level of the sensory cell bodies in the DRG to the distal axon. Dorsal root ganglions are a prominent target as they are less protected by the blood–nerve barrier and more vulnerable to neurotoxic damage, potentially explaining the predominance of sensory involvement in patients with CIPN. Platinum compounds form DNA adducts that accumulate in DRGs and lead to cell death in sensory neurons. Vinca alkaloids, taxanes, and thalidomide have also been associated with DRG damage [5]. Additionally, a “dying back” axon degeneration of distal nerve endings serves as the major pathology in this disorder [5].
The prevalence of CIPN is agent-dependent, with reported rates varying from 19% to more than 85% and is the highest in the case of platinum-based drugs (70–100%), taxanes (11–87%), thalidomide and its analogues (20–60%), and ixabepilone (60– 65%); so, in this review we focus on platinum-induced peripheral neuropathy [6].
On one hand, platinum-based drugs are subjected to uptake by the sensory neuron cells via passive diffusion through the plasma membrane, and on the other hand, active transport is necessary through the copper transporters OCT1, OCT2, and CTR1 for entry into the cell. Correspondingly, when platinum-based agents enter the neuron, they become reactive via aggressive hydration and can also respectively bind to nuclear and mitochondrial DNA regions. Platination of the nuclear DNA may cause increase in the expression of Ape-1 and pol K protein, leading to the occurrence of an inefficient DNA damage repair system (i.e., BER and NER pathways) and a decrease in general transcription. In parallel, Ape-1 protein also results in the activation of p53, following which activated p53 induces the release of cytochrome c (CytC) from the mitochondria and subsequent caspase-3 activation. All above mentioned phenomena may cause neuronal death due to apoptosis. Furthermore, the binding of platinum-based drugs to the mitochondrial DNA may induce the decrease of replication and lead to a failure in overall function at the mitochondrial level. This eventually causes depletion of ATP and an increase in ROS formation as well as sequestration of intracellular calcium. Notably, the mitochondria are considered the main sources of ROS production and are recognized as the major targets for ROS- induced oxidative damage, a phenomenon which can lead to the reduction of the efficiency of mitochondria and induction of apoptosis.
Oxaliplatin exposure can respectively affect the activity and kinetics of both voltage-gated sodium channels (VGSC) and voltage-gated potassium channels (VGKC). On one hand, oxaliplatin exposure can influence the functional properties of VGSC, resulting in hyperexcitability of dorsal root ganglia (DRG) sensory neurons; on the other hand, oxaliplatin exposure can also lead to functional abnormalities of VGSC and improve cell excitability by increasing hyperpolarization-activated channel (HCNs) expression. Additionally, the transcription levels of T- and L-type voltage-gated calcium channels (VGCC) increase after oxaliplatin exposure, resulting in the dysregulation of Ca2+ homeostasis. Oxaliplatin exposure also leads to an upregulation of the sensitization of the transient receptor potential cation channel subfamily V member 1(TRPV1), transient receptor potential cation channel subfamily a member 1 (TRPA1), and transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8) in cultured DRG neurons, and this occurrence plays a pivotal role in the neuronal hyperexcitability phenomenon [7].
Ziziphus jujuba Mill, a member of the family Rhamnaceae, commonly known as Annab, is used traditionally as tonic and aphrodisiac and sometimes as hypnotic-sedative and anxiolytic, anticancer (melanoma cells), antifungal, antibacterial, antiulcer, anti-inflammatory, cognitive, antispastic, antifertility/ contraception, hypotensive and antinephritic, cardiotonic, antioxidant, immunostimulant, and wound healing properties [8].
Various studies have shown that the jujube fruit contains many bioactive compounds, including triterpenic acids, flavonoids, cerebrosides, phenolic acids, α-tocopherol, β-carotene, and polysaccharides. Jujube fruit has more total phenolic compounds compared to other common fruits that exhibit antioxidant activities, such as cherry, apple, persimmon, or red grape. Flavonoids, phenolic acids, tannins, stilbenes, and lignans are derivatives of phenolic compounds [9].
Different parts of Z. jujuba were reported to have antioxidant, anti-inflammatory, hepatoprotective, anti-tumor and induction of erythropoietin expression activities [10]. polysaccharides and triterpenic acids as the bioactive compounds in jujube induce apoptosis and have shown anti-proliferative effects on cancer cell lines [11,12]. Polysaccharides also manifest antioxidant activities. In a study found that SAZMP4 as a novel antioxidant pectin isolated from jujube was composed predominantly of rhamnose, arabinose, xylose, mannose, and GalA through 1,4-linked GalA (93.48%) [13]. In recent years, LZJP3 and LZJP4 extracted from Z. jujuba cv. Linzexiaozao as polysaccharides component have strong antioxidant effects, as evaluated by DPPH radicals, hydroxyl radicals, hydrogen peroxide, and superoxide radicals [14].
Ursonic acid as triterpenoid component extracted from Z. jujube was mentioned as potential to tackle COVID-19 by inhibiting virus replication [15,16]. Alphitolic acid as anti-neuroinflammatory and noninhibiting activity of the group of triterpenoids is present in Z. jujube. The effects of aliphatic acid, an active constituent in the fruit of this plant, on NO inhibition were examined in LPS Factors which may induce NO production in Murine microglia (BV2) primary microglia and RAW cells induced and LPS and IFN-γ mediated microglia and macrophages’ activation and in a neuro- inflammation model, which suggested anti-neuroinflammatory properties [17].
Ahmed M Mesaik conducted preclinical study to discover the anti- inflammation poverty of Z. Jujuba given to rats administrated of Z. jujuba ethanolic extract (800 mg/kg, 1200 mg/kg or 1600 mg/kg) before injection of 0.1 ml carrageenan solution widely been used as an experimental animal model to evaluation of anti-inflammatory potential and some group took diclofenac sodium (100 mg/kg) as positive control and 2% tragacanth negative control solution orally. Among those doses, 1200mg/kg had maximum anti-inflammatory although all doses of Z. jujuba ethanolic extract exhibited lower anti-inflammatory activity than the positive control group and reduced the paw volume significantly (P≤0.001) from 1 h to 6 h. The mechanisms of action of Z. jujuba ethanolic extract may be similar to that diclofenac sodium whereby the anti-inflammatory effect observed might be due to the inhibition of expression and activity of COX-2. Based on the chemical analysis done, it was found that Z. jujuba fruit contains both betulinic acid and quercetin. Earlier studies showed that they inhibited the release of cytokine and induce the nitric oxide synthase via inhibition of the NF-κB pathway [18].
Another study, 200 and 400 mg/Kg of jujube extracts administrate to 6 rat showed Z. jujuba can reduce acute and chronic inflammation caused by carrageenan by reducing the expression of nitric oxide and prostaglandins (P < 0.05) The anti-edematous effect of Z. jujuba was significantly high during all the stages of inflammation, indicating the inhibition of release of COX, histamine and nitric oxide (NO) [19].
In vivo experimental study Ziziphus jujuba cv. Jinsixiaozao attenuated acetaminophen-induced inflammatory mediator production in Male BALB/c mice, such as NO, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β). Expression of p65 after administration of 70% aqueous ethanol 500gr powder Ziziphus jujuba cv. Jinsixiaozao dampened nuclear factor-κB (NF-κB) activation. The results strongly indicate that the hepatoprotective role of Ziziphus jujuba cv. Jinsixiaozao in acetaminophen-induced hepatotoxicity might result from its induction of antioxidant defense via activation of Nrf2 and reduction of inflammation via inhibition of NF-κB [20].
Accordingly, In vivo antioxidant studies were reported that Drosophilas fed with 150 mg/mL jujube fruit supplement could effectively reduce ROS stress and increase their average survival time. It also extends their lifespan [21].
Many studies supported the neuroprotective ability of jujube.
jujuba activates choline acetyltransferase and may alleviate symptoms of Alzheimer’s disease; so, a study effort to investigate the impact of jujube in rat model of Alzheimer’s disease (AD) at the doses 500 and 1,000 mg/kg/ day for 15 days. Finally, jujube had shown the repairing effect on behavioral and memory disorders caused by nucleus basalis of Meynert lesion in rats [22].
jujuba also can be used as anti-depressant according results of behavioral tests like a tail-suspension test (TST), forced swimming test (FST) and open field test, and applied chronic unpredictable mild stress test in mice revealed [23]. In other study Flavonoids and saponins extracted from the seed of Z. jujuba have manifested hypnotic and sedative effects [24].
Various plant extracts have been screened and investigated for their potential neuropharmacological activities in different experimental models of animals comprising mice and rats. Herbal extracts and natural products including Z. jujuba can be used alone or as adjuncts to standard drugs, used for various neurological diseases like sedative and hypnotic, anxiolytic and antiseizure [25,26]. jujuba fruit suppressed intestinal inflammation by blocking the NF-κB/IL-6/JAK1/STAT3 pathway in colorectal cancer in mice. They investigate the protective effect of Z. jujuba on the NF-κB/IL-6/JAK1/STAT3 signaling pathway evaluated the activation of proteins involved in the signaling. Triterpenic acids in Z. jujuba fruits are the active components responsible for the anti-inflammatory and anticancer activities by dietary Z. jujuba attenuated inflammation, tumor development and progression by downregulation of expression of IL-6, STAT3, and NF-κB. It also increased Bax, proapoptotic and Bcl-2, anti-apoptotic protein expression [27].
The double-blind clinical trial was performed among 100 breastfeeding women. It was advised to apply 0.5 mL of Z. jujuba Fruit lotion (210 mg of the juice of Z. jujuba fruit) with 54% efficiency was made by using 96%-ethanol on the nipple and areola five times a day after each breastfeeding [28].
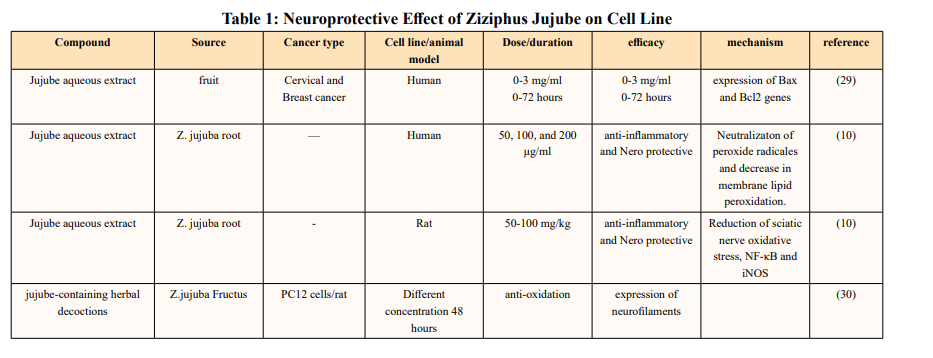
Neuroprotective Effect of Ziziphus Jujube in Cell Lines Abedidini and his coworkers have shown jujube aqueous extract invitro has anti-cancer and pro-apoptotic abilities of cervical and breast human cancer cell lines [29].
Raghuram Kandimalla and et al. evaluated the effect of jujube fractions on H2O2 intoxicated SHSY5Y cell lines and DRG neurons. Their culture media from all the treatment groups were collected after the herbal treatment period to measure the LDH levels. They further induced diabetic neuropathy (DNP) with streptozotocin (55 mg/kg) in male Wister rats to test the active fractions of jujube. Body weight changes, blood glucose levels and pain threshold through hot plate, tail immersion, cold plate and Randall-Sillitto methods were performed on 0th, 4th, 6th, and 8th week of the study. After completion of the herbal treatment period, all the animals were sacrificed to measure the sciatic nerve lipid peroxidation, antioxidative enzyme levels (SOD, catalase, and GSH) and cytokine levels (IL-1β, IL-6, IL-10, TNF-α, iNOS, and NFκB) through ELISA and western blotting analysis. Pain is the main symptom of DNP, which is characterized by thermal, mechanical hyperalgesia and cold allodynia. Hyperglycemia leads to Activated polyol pathway advanced glycation end products (AGEs) cause receptor-mediated oxidative stress and stimulate NF-κB protein complex which is capable of activating an inflammatory response. Apart from proinflammatory cytokines, anti-inflammatory cytokine IL-10 play a crucial role in pathogenesis of DNP. Nerve injury causes the significant reduction of IL-10 in DNP patients. ZJWF treatment potentially inhibited NF-κB, iNOS expression and related proinflammatory cytokines in sciatic nerve of DNP rats and showed increase levels of IL-10. Z. Jujuba also neutralizes the ROS and provided the protection against oxidative damage and inflammation to sciatic nerve cells [10].
Lam CTW and et al. demonstrated that aqueous extract of mature jujube fruit treatment promotes the protein expression of neurofilaments like NF68, NF160 and NF200. The pre-treatment of herbal extracts protected PC12 cells against oxidative stress- induced apoptosis in a dose-dependent manner. Moreover, the herbal treatments triggered the mRNA expressions of relevant anti-oxidation genes, i.e. glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modulatory subunit (GCLM), glutathione S-transferase (GST) and NAD(P)H quinone oxidoreductase (NQO1) via the activation of anti-oxidant response element (ARE) [30].
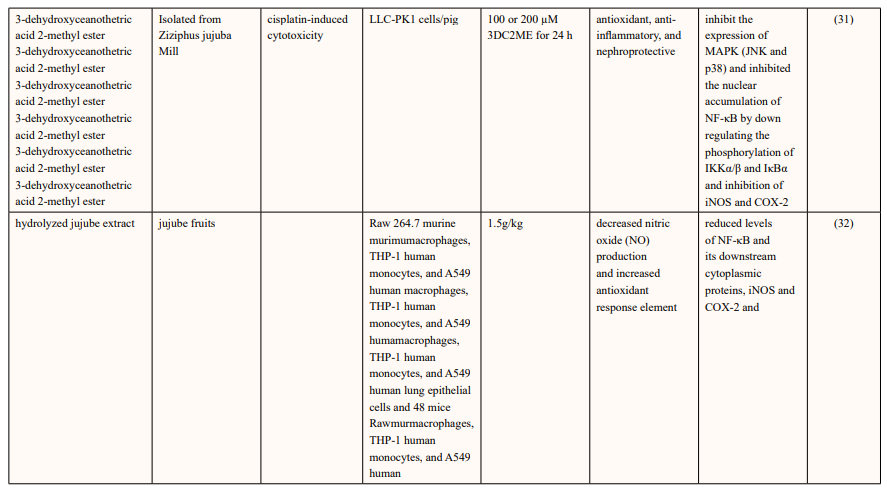
They investigated the antioxidant and anti-inflammatory effects of 3-dehydroxyceanothetric acid 2-methyl ester (3DC2ME) isolated from Z. jujuba Mill. In LLC-PK1 cells following cisplatin-induced cytotoxicity. the increase in the expressions of IκB kinase α/β (IKKα/β), inhibitor of kappa B alpha (IκBα), nuclear factor kappa B (NF-κB), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in these cells was inhibited. In addition, pretreatment with 3DC2ME upregulated heme oxygenase 1 (HO-1) via the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in the cisplatin-treated LLC-PK1 cells. These results provide basic scientific evidence for understanding the antioxidant and anti-inflammatory effects of 3DC2ME isolated from Z. jujuba against cisplatin-induced kidney epithelial cell death [31].
The hydrolyzed jujube extract, contained higher levels of quercetin, total phenolics, and flavonoids, and exhibited more effective radical-scavenging abilities in comparison to non- hydrolyzed jujube extract. The hydrolyzed jujube extract treatment decreased production of inflammation-associated molecules, including nitric oxide and pro-inflammatory cytokines and also reduced expression of NF-κB and its downstream proteins in A549 human lung epithelial cells. Moreover, oral supplementation of 1.5 g of hydrolyzed jujube extract per kg of body weight (BW) attenuated histological lung damage, decreased plasma cytokines, and inhibited expression of inflammatory proteins and oxidative stress mediators in the lungs of mice exposed to benzo(a)pyrene at 50 mg/kg BW. Expression levels of antioxidant and cytoprotective factors, such as nuclear factor erythroid-derived 2-related factor 2 and heme oxygenase-1, were increased in lung and liver tissues from mice treated with hydrolyzed jujube extract, compared to mice fed to non-hydrolyzed jujube extract [32].
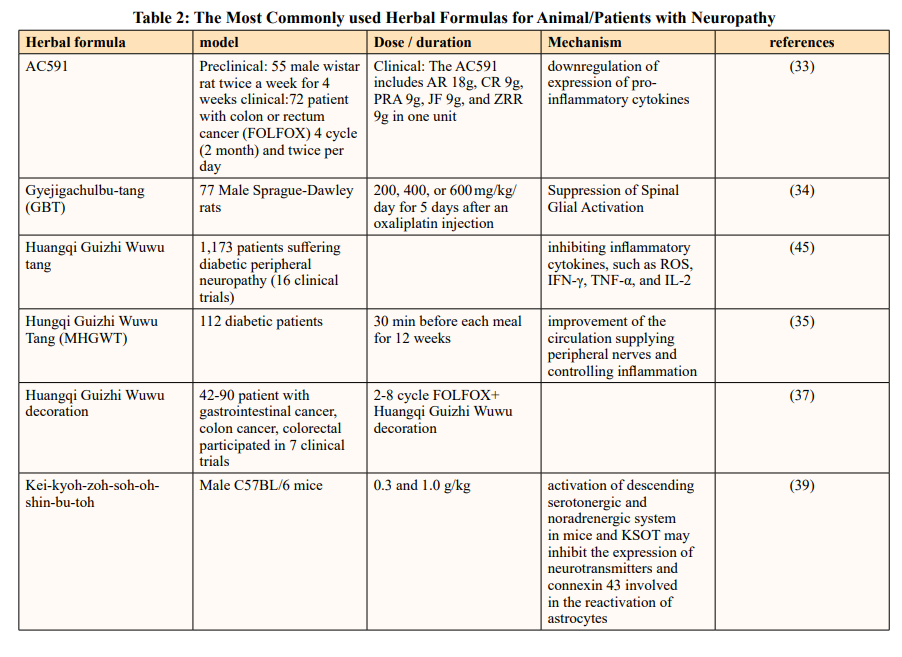
Various studies also exhibit Z. jujube has potential neuroprotective effect in vitro experiments and in vivo studies. Cheng et al. explored the neuroprotective strategies of AC591 in animal models of oxaliplatin-induced neuropathy. AC591 is a standardized extract of Huangqi Guizhi Wuwu decoction (HGWD, Ogikeishigomotsuto, in Japanese), which consists of Astragalus mongholicus, Cinnamomum cassia, Paeonia lactiflora Pall. Fruit of Z. jujuba Mill and Zingiber acuminatum Valeton. They found that after AC591 pretreatment in animal models, events of oxaliplatin-induced cold hyperalgesia, mechanical allodynia, and morphological damage were decreased. This protective function largely relies on the modulation of multiple molecular targets and pathways that participate in the downregulation of expression of pro-inflammatory cytokines (such as IL-1β, IL-6, and TNF-α). Xiaolan Cheng and et al. designed a study that 72 patients were randomly participated to treatment in the AC591 and Non-AC591 groups. Patients in AC591 group were scheduled to receive at least four cycles (2 months) of oxaliplatin-based FOLFOX chemotherapy plus AC591. The AC591 includes 18 g Astragali Radix, 9 g Cinnamomi ramulus, 9 g Paeonia radix alba, 9 g Jujubae fructus, and 9 g Zingiberis rhizoma in one unit. All patients in the AC591-treated group were asked to take AC591 twice daily (in the morning and in the evening) for a total of 54 g crude drug/ day. The severity of peripheral neuropathy was evaluated and graded into five categories according to the oxaliplatin-specific Levi’s scale. They finally realized that AC591 ameliorated the oxaliplatin-induced neuropathy in colorectal cancer patients [33].
Another study also demonstrated Gyejigachulbu-tang (GBT) (Gui-Zhi-Jia-Shu-Fu-Tang, Chin. Keishikajutsubuto, Jap.) as herbal formula containing Cinnamomum cassia, Paeonia lactiflora, Atractylodes lancea (Thunb.) DC. Fruit of Z. jujuba, Glycyrrhiza uralensis Fisch. Zingiber acuminatum, and Aconitum carmichaelii used to effectively relieve oxaliplatin-induced acute cold and mechanical hypersensitivity. In east Asian countries, such as Korea, Japan, and China, GBT has been widely used to treat various pain symptoms. Ahn et al. suggested that GBT has a protective effect was achieved via prevention of the activation of spinal astrocytes and microglia, as well as restoration of immune activities of glial fibrillary acidic protein (GFAP) and OX42 (microglia marker) [34].
Hungqi Guizhi Wuwu Tang is composed of the following seven species: Astragali Radix, Cinnamomum cassia, Paeonia lactiflora, Zingiber officinale, Z. jujube, Spatholobus suberectus and Pheretima aspergillum. In a randomized, double-blind, and placebo- controlled trial, a Chinese medicinal formula, modified Hungqi Guizhi Wuwu Tang (MHGWT), was found to be effective and well tolerated in diabetic patients with DPN. The MHGWT regimen reduced the pain and numbness of extremities and improved quality of life during the 12 weeks of treatment Participants were asked to take one pack (4 g MHGWT or placebo) three times daily, 30 min after breakfast, lunch, and dinner, throughout 12 week of the treatment [35]. Other Clinical investigations have suggested that it has therapeutic potential for DPN [36].
In another study demonstrate Huangqi Guizhi Wuwu Decoction is neuroprotective against Oxaliplatin-induced Peripheral Neurotoxicity in Cancer patients. They mentioned that 7 randomized clinical trial used Huangqi Guizhi Wuwu Decoction in different type of cancer like gastrointestinal cancer, colon cancer, colorectal cancer base FOLFOX regime between 45 -90 patient participant [37,38].
Kei-kyoh-zoh-soh-oh-shin-bu-toh (KSOT) is a traditional herbal formulation consisting of seven herbal medicines: Cinnamomi cassia, Zingiber officinale, Ziziphus jujuba Glycyrrhiza Ephedra sinica, Asiasarum sieboldii and Aconitum carmichaeli. Scientists investigated the effects of KSOT on mechanical allodynia induced by chemotherapeutic drugs (oxaliplatin, paclitaxel, vincristine, and bortezomib) in mice. In addition, they also investigated the mechanisms underlying the antiallodynic action of KSOT. KSOT was given once at the peak of mechanical allodynia induced by each chemotherapeutic drug. A single administration of KSOT (1.0 g/kg) did not inhibit mechanical allodynia induced by oxaliplatin, paclitaxel, vincristine, or bortezomib at least for the period evaluated. Prophylactic repetitive oral administration of KSOT (0.3 and 1.0 g/kg) significantly inhibited the exacerbation of mechanical allodynia from day 9 after oxaliplatin injection, compared to that observed in the vehicle-treated group. However, KOST (0.3 and 1.0 g/kg) did not affect the mechanical allodynia induced by paclitaxel, vincristine, or bortezomib. In this study, a single oral administration of KSOT did not affect the mechanical allodynia induced by the studied chemotherapeutic agents. On the other hand, prophylactic repetitive oral administration of KSOT inhibited the exacerbation of mechanical allodynia induced by oxaliplatin but was not effective in the case of paclitaxel, vincristine, and bortezomib. In addition, repetitive KSOT did not affect oxaliplatin-induced cold dysesthesia. These results suggest that prophylactic repetitive oral administration of KSOT is effective for oxaliplatin-induced mechanical allodynia. the antiallodynic action of KSOT in mice treated with chemotherapy drugs remain unclear [39].
In 30 male Wister rats, diabetes mellitus was induced by a single injection of STZ and each rat receiving Txl ultrafine powder 500 mg/kg/d Txl contains 12 medicinal components one of them is jujube.
The upstream transcriptional regulator related to mitochondrial biogenesis is PGC-1α, which increases COX IV and cytochrome c protein levels as well as the steady-state level of mtDNA. PGC-1α coordinately regulates gluconeogenesis, glycolysis, lipogenesis, peroxisomal and mitochondrial fatty acid oxidation, and mitochondrial respiration efficiency. However, the change of PGC-1α in diabetic peripheral nerves is unclear. In this study, they found a decrease of PGC-1α expression in sciatic nerves of diabetic rats, which may be related to the downregulation of COX IV and SOD expression and malfunction of mitochondrial biogenesis. This malfunction was reversed by Txl treatment.
Pharmaceutical analysis has demonstrated that ginsenoside Rg1, ginsenoside Rb1, paeoniflorin, jujuboside A, and jujuboside B are main active components in Txl [40]. Txl is first proved by the State Food and Drug Administration of China for angina pectoris and ischemic stroke treatment and also has been demonstrated as beneficial for diabetic complications due to its multiple biologic effects [41]. The region most affected by diabetic neuropathy is located in Schwann cell rich sciatic nerves, where striking upregulation of mitochondrial oxidative phosphorylation occurs [42]. The expression of PGC-1α, SOD, and COX IV of sciatic nerves was reversed by Tongxinluo treatment in DM+Txl group.
Impaired mitochondria are the main source of reactive oxygen species (ROS) [43]. Txl has been proven to provide protective effects on peripheral nerves due to its induction of neuron growth factors and inhibition of nerve apoptosis through the MAPK pathway, which is associated with increased accumulation of ROS. Here we also proved that Txl increases PGC 1α expression and therefore participates in mitochondrial biogenesis and energy metabolism; this finding may further illustrate the pleiotropic effect of the medicine. In conclusion, Txl can apparently improve the decreased mechanical allodynia and sciatic-tibial nerve conductive velocity and alleviate nerve impairment of diabetic rats [44].
The flavonoid quercetin is an active ingredient Huangqi Guizhi Wuwu tangs that concist of 5 plant one of them is jujube. Pang et al. conducted a meta-analysis of sixteen randomized controlled trials with a total of 1,173 patients suffering diabetic peripheral neuropathy. The results revealed that Huangqi Guizhi Wuwu tang had significant therapeutic efficacy in treating peripheral diabetic neuropathy [45]. Previous studies have shown that quercetin has various biological actions, such as antioxidative and anti- inflammatory effects [46]. including inhibiting inflammatory cytokines, such as ROS, IFN-γ, TNF-α, and IL-2 [47]. Quercetin suppresses the secretion of inflammatory cytokines by regulating transcription factors (NF-κB) [48]. Z. jujuba also has indicated the high probability of increased survival, tumor response to chemotherapy. Preclinical and clinical studies have pointed out that PHY906 as traditional Chinese formula can enhance the therapeutic indices of a broad spectrum of anticancer agents. PHY906 is composed of a decoction of a mixture of the four herbs jujuba, Paeonia lactiflora, Glycyrrhiza uralensis, and Scutellaria baicalensis. Preclinical studies on various mouse tumor xenograft and allograft models have shown that PHY906 enhances the therapeutic indices of a broad spectrum of anticancer agents. The PHY906 clinical program consists of five trials in three different types of cancers, colorectal, liver, and pancreatic cancers, in both the United States and Taiwan. A total of 150 patients received PHY906 administered orally as 200 mg capsules in various dose regimens [49].

In conclusion, Z. jujube can be used in treatment of CIPN; but further investigation is needed to find out that how is the most effective way of using this herb in CIPN; which formulation, which strength, how many times in a day or a week and etc. Other pharmacological properties of Z. jujube, such as anticancer and anti-drug resistance in cancer, makes it more attractive to use in combination with chemotherapy agents due to its potential.
None.
The authors declare that there is no conflict of interest.
