Author(s): <p>Anubha Bajaj</p>
Congenital granular cell epulis is an uncommon, benign lesion preferentially arising in neonates. Initially scripted by Neumann in 1871, congenital granular cell epulis is additionally designated as congenital granular cell tumour, congenital granular cell myoblastoma, granular cell epulis of infancy, granular cell fibroblastoma or congenital epulis [1]. Congenital granular cell epulis represents as a soft lesion arising within the oral cavity, predominantly the mucosa of maxillary alveolar ridge or mandibular alveolar ridge. Enlarged lesions may engender dysphagia, difficulty in feeding, respiration and aberrant mouth closure. Of undetermined histogenesis, diverse postulates regarding origin of congenital granular cell epulis generally lack consensus.
Congenital granular cell epulis is an uncommon, benign lesion preferentially arising in neonates. Initially scripted by Neumann in 1871, congenital granular cell epulis is additionally designated as congenital granular cell tumour, congenital granular cell myoblastoma, granular cell epulis of infancy, granular cell fibroblastoma or congenital epulis [1]. Congenital granular cell epulis represents as a soft lesion arising within the oral cavity, predominantly the mucosa of maxillary alveolar ridge or mandibular alveolar ridge. Enlarged lesions may engender dysphagia, difficulty in feeding, respiration and aberrant mouth closure. Of undetermined histogenesis, diverse postulates regarding origin of congenital granular cell epulis generally lack consensus.
Congenital granular cell epulis predominantly appears within the neonates. A female predominance is observed with a female to male proportion of nearly 10:1. Anterior mucosal maxillary alveolar ridge preferentially exhibits the neoplasm with the proportion of around ~2-3: 1, in contrast to mucosa of mandibular alveolar ridge wherein the lesion is precisely situated upon the canine or lateral incisor. Tumefaction may enlarge within the prenatal phase, especially the third trimester, although tumour progression ceases following birth [2, 3].
Congenital granular cell epulis usually appears as a solitary lesion whereas multiple lesions appear in an estimated 10% subjects. Multifocal lesions commonly incriminate the maxilla and mandible.
Of obscure pathogenesis, the neoplasm is posited to arise from Schwann cells, fibroblasts, mesenchymal cells or undifferentiated cells. Besides, around 20% tumefaction may emerge from odontogenic epithelial rests [2, 3].
Also, it is hypothesized that congenital granular cell epulis is meticulously associated with maternal hormones although is devoid of oestrogen and progesterone receptors [2, 3]. Additionally, stromal macrophage infiltration induces spontaneous retrogression and transformation of the fibrous neoplasm. Thus, the lesion may be contemplated to be of reactive or degenerative origin.
Genesis of interstitial cells remains undetermined. However, it is postulated that interstitial cells display a neuroendocrine differentiation or represent a preliminary stage of granular cells [2, 3].
An association with syndromic activity and genetic deformities is absent. Malignant metamorphosis remains undocumented [2, 3].
Congenital granular cell epulis manifests as a well circumscribed, soft to firm, pink or normal hued, sessile or pedunculated mucosal lesion with a smooth, non ulcerated superficial surface which is generally situated upon the maxillary alveolar ridge. Congenital granular cell epulis usually represents with a polypoid mass with a broad or thin base, magnitude varying from 0.5 centimetres to 2 centimetres and a superimposed, pinkish or erythematous mucosa. Nevertheless, enlarged masses of up to ~ 9 centimetre diameter are documented [4, 5].
Ulcerated superimposed mucosa may engender difficulties in feeding, breathing or closure of mouth. The lesion may hinder breast feeding in incriminated neonates. Occasionally, the neoplasm undergoes spontaneous retrogression or transformation within first year of life on account of macrophages infiltrating neoplastic stroma.
Upon gross examination, a singular, well circumscribed, whitish, polypoid or nodular lesion of variable magnitude and pinkish mucosal surface is observed. The lesion may appear as a confluent, nodular, submucosal tumour expanse. Cut surface is smooth, glistening and tan or yellow. Typically, congenital granular cell epulis exhibits proliferating sheets, expansive nests or bands of monomorphic, enlarged, polygonal to mildly spindle-shaped cells imbued with an abundant, eosinophilic, granular cytoplasm, bland, eccentric nuclei and discernible nucleoli [4, 5].
Angulated interstitial cells, cytoplasmic hyaline globules, odontogenic rests, distended vascular channels and scattered, chronic inflammatory cells as mature lymphocytes appear intermingled with polygonal tumour cells. Infrequently, the circumscribing, delicate stroma may be incorporated with chronic inflammatory cells such as lymphocytes and macrophages [4, 5].
Superimposed stratified squamous epithelium is attenuated or atrophic and lacks prominent rete ridges or foci of pseudoepitheliomatous hyperplasia [4, 5].
Spindle-cell variant of congenital granular cell epulis characteristically exhibits a predominance of spindle-shaped cells and an absence of typical granular cells. Superimposed stratified squamous epithelium demonstrates acanthosis and broad rete ridges [5, 6].
Tumour cells are immune reactive to vimentin or acid phosphatase. Nearly 40% neoplasms are immune reactive to neuron specific enolase (NSE) [5, 6].
Congenital granular cell epulis is immune non-reactive to S100 protein, calponin, laminin, CD31, CD34, CD68, NGFR/p75, inhibin-alpha, Leu-7, chromogranin, nerve growth factor receptor (NGFR), desmin, keratins, smooth muscle actin or GLUT-1. Proliferation index as determined by Ki-67 and immunostaining with proliferating cell nuclear antigen (PCNA) varies between roughly 11% to 33.3% [5, 6].

Figure 1: Congenital granular cell epulis exhibiting a sessile, reddish, expansive tumefaction with a smooth, non-ulcerated surface.

Figure 2: Congenital granular cell epulis delineating sheets of polygonal cells with abundant, eosinophilic cytoplasm and uniform nuclei enveloped within a fibrotic stroma.

Figure 3: Congenital granular cell epulis exemplifying nests of polygonal cells imbued with abundant, granular cytoplasm and uniform nuclei with stromal lymphocytic infiltration

Figure 4: Congenital granular cell epulis displaying polygonal cells with abundant, eosinophilic cytoplasm, bland nuclei and stromal lymphocytes.
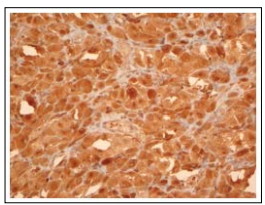
Figure 5: Congenital granular cell epulis demonstrating immune reactivity to CD68
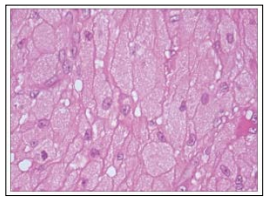
Figure 6: Congenital granular cell epulis depicting polygonal cells with abundant, eosinophilic cytoplasm, bland, uniform nuclei and surrounding fibrosis
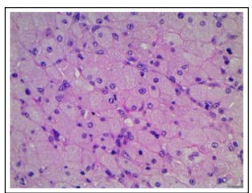
Figure 7: Congenital granular cell epulis enunciating polygonal cells with abundant, granular cytoplasm, uniform nuclei and stromal lymphocytes.
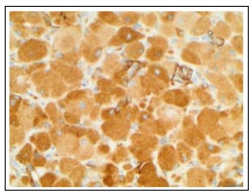
Figure 8: Congenital granular cell epulis exhibiting immune non reactivity to laminin
Oral masses discerned in foetuses and neonates require a distinction from congenital granular cell epulis. Upon morphological assessment, the neoplasm simulates adult granular cell tumour (GCT) which typically arises upon the tongue of adults between 30 years to 60 years and exhibits a female to male proportion of nearly ~2:1. The neoplasm is composed of nests or bands of proliferating polygonal, granular, eosinophilic cells with occasional intracytoplasmic hyaline globules. Tumour cells are typically immune reactive to neuron specific enolase (NSE), CD68 and S100 protein. Besides, granular cell tumour frequently occurs upon the soft palate or floor of the mouth. Furthermore, superimposed stratified squamous epithelium exemplifies pseudoepitheliomatous hyperplasia [7, 8].
The exceptional granular cell tumour is immune non-reactive to S100 protein. Also, primitive polypoid non-neural granular cell tumours of the oral cavity are also documented [7, 8]. Neoplasms composed of cells with abundant granular, eosinophilic cytoplasm are characteristic of diverse lesions of infancy such as
• soft tissue odontoma which exhibits an abundant fibrous and myxoid stroma admixed with dentin, enamel and pulpal elements [7, 8].
• neuroectodermal tumour of infancy is comprised of enlarged, peripheral cells immune reactive to S100 protein, human melanoma black 45 (HMB-45) antigen and cytokeratin whereas miniature neuroplastic cells are immune reactive to S100 protein, glial fibrillary acidic protein (GFAP) and synaptophysin [7, 8].
Clinical segregation of congenital granular cell epulis is required from neoplasms such as infantile myofibroma, rhabdomyoma, melanotic neuroectodermal tumour of infancy, peripheral odontogenic fibroma, verruciform xanthoma and neurofibroma. Occurrence of features such as spindle-shaped cells and extensive fibrosis may indicate a primary rhabdomyoma or infantile myofibroma. •rhabdomyoma typically incriminates cardiac muscle of adult males. Neoplasms within the head and neck manifest as distinctive extra-cardiac lesions. The foetal subtype is composed of spindle-shaped cells whereas the adult subtype is composed of polygonal cells imbued with granular, eosinophilic cytoplasm. Infantile myofibroma incriminates the tongue or mandible. The neoplasm represents a proliferation of spindle-shaped cells intermingled with hemangiopericytoma-like vascular areas. The spindle-shaped cells are immune reactive to actin [7, 8].
Upon radiographic imaging, features simulating a cystic, lymphatic or angiomatous lesion are usually absent and the images may recapitulate a bone fibroma [9, 10].
Congenital granular cell epulis can be appropriately detected in utero through antepartum ultrasonography or magnetic resonance imaging (MRI). The gradually evolving tumefaction commonly manifests within third trimester of pregnancy [9, 10].
Ultrasonography may enhance proportionate prenatal disease discernment and is optimal for therapeutic planning with pertinent surgical approach [9, 10].
Upon computerized tomography (CT) and magnetic resonance imaging (MRI), a poly-lobed lesion of variable magnitude is observed [9, 10].
Upon T1-weighted imaging, a minimally hyper-intense lesion is exemplified. Upon T2-weighted imaging a heterogeneous, mildly hyper-intense neoplasm is delineated. Generally, continuity of the lesion with palatine bone or palatoschisis is absent [9, 10].
Upon ultrasonography and magnetic resonance imaging, cystic lesions characteristically depict an anechoic, centric zone concordant to fluid content [9, 10].
Congenital granular cell epulis can be appropriately alleviated with comprehensive surgical excision extending to the periosteal layer. Surgical intervention is necessitated in order to initiate appropriate breast feeding in incriminated neonates [9, 10].
Conservative treatment option with extensive monitoring can be adopted for miniature lesions of congenital granular cell epulis [9, 10].
Following cogent surgical manoeuvres, congenital granular cell epulis exhibits an excellent prognosis [9, 10].
Tumour reoccurrence following adequate surgical extermination is uncommon [9, 10].
Table: Differential Diagnosis of Congenital Granular Cell Epulis [2].
| Lesion | Features | Site | Morphology | IHC |
| Congenital granular cell epulis | Neonate F>M | Alveolar ridge of maxilla | Sheets of polygonal, eosinophilic, granular cells with thin superimposed squamous epithelium | Vimentin +, S100- |
| Granular cell tumour | 30 years to 60 years F>M | Tongue | Sheets of polygonal, eosinophilic, granular cells with pseudoepitheliomatous hyperplasia of stratified squamous epithelium | S100+, CD68+ |
| Rhabdomyoma | Extracardiac in adults M>F | Head and neck is a common extracardiac site. | Foetal subtype- myocyte differentiation Adult subtypepolygonal eosinophilic cells with granular cytoplasm and cross striations | Desmin+, SMA+ |
| Infantile myofibroma | Neonate to 6 years M>F | Tongue and buccal mucosa | Nodular, biphasic proliferation of plump, peripheral myofibroblasts and centric, round to spindle hyperchromatic cells and haemangiopericytoma-like areas | Vimentin+, SMA+ |
| Melanotic neuroectodermal tumour of infancy | Median at 5 months. F=M | Maxilla | Admixed miniature neuroblastic cells and large, melanin containing cells | Epithelial cellsCK+,Vimentin+, EMA+,HMB-45+. Small neuroblastic cells synaptophysin+, NSE+ |
| Peripheral odontogenic fibroma | Neonates to 80 years. F>M | Mandible | Cellular connective tissue with multiple small islands and strands of odontogenic epithelium | Epithelial cells CK+ |
IHC-Immunohistochemistry, CK-Cytokeratin, SMA-Smooth muscle actin, EMA-Epithelial membrane antigen, NSE-Neuron specific enolase, HMB-45-Human melanoma black antigen 45.
