Author(s): Moore Ikechi Mike-Ogburia*, Barynem Vito-Peter, Gift Mibilanyeofori Hart, Aisha Dio, Victory Chidinma Nwogu, Ozioma Chiagoziem Okoro, Okadini Collins Albert, Patmos Obu Angala, Miracle Chinemerem Clifford, Priscilla Adonike Ogbakiri, and Chiziyara Orluibna Obunwo
Infectious diseases like HIV and syphilis are a significant burden in prisons, where they are more prevalent than in the general population. The confined unhygienic living conditions and high-risk behaviours contribute to the spread of infections among incarcerated individuals. This study was therefore aimed at investigating the prevalence of HIV and syphilis among inmates incarcerated in Port Harcourt, Nigeria. The study employed a descriptive cross-sectional study design involving 200 inmates from the Port Harcourt Maximum Security Custodial Centre. A well-structured questionnaire was administered in addition to screening for HIV-1/2 antibodies as well as antibodies against Treponema pallidum using rapid diagnostic test kits. Descriptive statistics and chi-square were performed on the GraphPad Prism 9 software with a p-value of less than 0.05 considered statistically significant. The seroprevalence of HIV reported in this study was 6.5% while 4.5% was reported for syphilis with an HIV-syphilis co-infection of 0.5%. The duration of incarceration, history of blood oath, transactional sex and sharing of personal belongings were significantly associated with the prevalence of HIV while intravenous drug use, sharing of personal belongings and homosexuality were significantly associated with the prevalence of syphilis (p < 0.05). The findings of this study necessitate further research and the implementation of interventions targeting specific high-risk populations like prisoners. Offering HIV and syphilis testing and treatment, mitigation of unsafe sex and injection drug use practices as well as improvement of hygiene and sanitation in prison facilities are strongly recommended for limiting the spread of HIV and syphilis among the incarcerated.
Infectious diseases continue to be a major global health burden, affecting different populations in both developed and developing countries [1-3]. Despite advances in medical science and technology, infectious diseases continue to cause significant morbidity and mortality, particularly in low- and middle-income countries [4,5]. Sexually transmitted infections (STIs) impose a significant impact on sexual and reproductive health in developing nations like Nigeria [6,7]. Although more than thirty infections are known to be sexually transmitted, only eight have been linked to a high incidence of morbidity and mortality, including syphilis and Human Immunodeficiency Virus (HIV). While syphilis is presently treatable, HIV infection has neither a cure nor a vaccine, but there are antiretroviral medications available for its management [8]. Since the turn of the millennium, the projected global incarcerated population has grown by around 24.3%, with approximately 10.7 million individuals incarcerated in different prisons in the world [9]. The spread of infectious diseases among inmates is facilitated by the accumulation of negative health risks and precarious living circumstances in prisons, such as a sedentary lifestyle, poor diets, insufficient hygiene practices, and drug usage [10]. In prisons, high-risk transmission behaviours such as continued drug injection and syringe sharing, risky sexual conduct, tattooing, and piercing may result in rapid and severe disease transmission and development [11,12].
The number of incarcerated individuals in Nigeria is estimated to exceed 68,000, which surpasses the actual capacity of the prisons by more than 20% [13]; the Nigerian prison system faces many challenges, including overcrowding, poor conditions, and limited access to healthcare services, which can contribute to the spread of disease. There are more than 148 prisons in Nigeria, and overcrowding is a typical occurrence in these facilities [14]. Originally intended for 804 convicts, the Port Harcourt prison which is well over 100 years old presently accommodates up to 5,000 detainees, including women and minors, with as much as 3,700 awaiting trial [15].
HIV and syphilis are sexually transmitted infections of viral and bacterial agents respectively that continue to affect populations globally. Among prisoners, the prevalence of these infections is significantly higher compared to the general population [16]. Infection with HIV has been shown to be prevalent among inmates globally [10,17], within the African continent [18,19], and in Nigeria as well [20-22], with prevalence rates ranging from 2.76% in Northern Nigeria to 3.9% in Southern Nigeria [20,22]. Studies have also shown that a higher burden of syphilis exists among prisoners than in the general population [23,24], reporting a prevalence of 2.89% [25], to as high as 11.6% [23], with an even higher prevalence of 31.7% reported in a Pakistani study [26]. This recorded prevalence of HIV and syphilis can be attributed to several factors, including the high rate of unprotected sexual contact, limited access to healthcare services, and inadequate public health interventions. Despite the epidemiology of syphilis in Nigeria being extensively studied [27-29], there remains a paucity of literature on the prevalence of syphilis amongst the incarcerated population in the country, particularly in Port Harcourt.
The high prevalence of syphilis among prisoners is a major public health concern, as it not only affects the health of individual prisoners but also has implications for public health beyond the prison walls, as the risk of transmission to the wider community increases upon release. Studies have hypothesized that released inmates may account for half of all new HIV diagnoses among persons who inject drugs in Eastern Europe and Central Asia [30]; these models may also be applicable in the Nigerian setting. By virtue of the fact that identification and early treatment of infected incarcerated individuals are crucial not only to minimize disease burden and expenses with the correctional facilities but also to decrease the potential of disease transmission to the general community following release, this study was therefore aimed at investigating the prevalence of HIV and syphilis among inmates incarcerated in Port Harcourt, Nigeria.
A descriptive cross-sectional design with multi-stage sampling was used in the study which was conducted from July to December 2022. Prior to further stratification, inmates were divided into male and female groups. Males were assigned random numbers for systematic random sampling, while females, who were fewer than their male counterparts, were selected from all eligible candidates. The beginning number was chosen at random, and the sampling interval was set at 5.
The study was carried out at the Port Harcourt Maximum Security Custodial Centre which is located in the Port Harcourt City Council Area of Rivers State, Nigeria (Figure 1). The number of prisoners varied around the 4,000 mark during the period of the research, as new prisoners were either brought in or discharged. About 109 square kilometres of land make up the entire region. Inmates from various criminal backgrounds, including those awaiting trial, convicted and those who have been sentenced to death, are remanded in this facility [31]. It is a prison designed for male and female inmates, with around 85% of the space designated for male inmates and roughly 15% of the space designated for female inmates [32].
Figure 1: Map of Nigeria and Rivers State, highlighting the study area [33]
The study population were incarcerated individuals in the Port Harcourt Maximum Security Custodial Centre, Port Harcourt. These groups of individuals are faced with many challenges, including overcrowding, poor conditions, and limited access to healthcare services, which can contribute to the spread of disease. The sample size was calculated based on the expected prevalence of HIV infection among inmates in Maximum-Security Prison, Port Harcourt as reported by Jeremiah et al. [22], which revealed a prevalence of 3.9% as follows.

The sample size was however increased to 200 subjects to account for anticipated drop-out or missing data while carrying out the research, and as well increase the power of the study. Hence two hundred (200) inmates were enrolled in this study.
Incarcerated subjects who gave informed consent were recruited while subjects who were not present at the time of data collection as well as those incarcerated subjects who withheld consent were excluded.
A structured questionnaire was interview-administered to participating prison inmates to obtain socio-demographic and behavioural data. The questionnaire was structured to bear minimal identity as names were not included, rather, serial numbers were used to tally test results and subjects to ensure confidentiality. The serial number on each questionnaire was duplicated on the sampling bottle for subsequent coupling of the result and subject.
Five millilitres (5 ml) of blood was collected from each subject and dispensed into a sterile plain container and allowed to clot. The serum obtained was used for serological diagnosis of antibodies to HIV-1/2 and Treponema pallidum.
Abbot Determine™ HIV -1/2 immunochromatographic rapid test kit (Abbott Laboratories, Abbott Park, IL, USA), visually read for the qualitative detection of antibodies to HIV-1/2 was used. Treponemal antibody was detected with the ACON rapid VDRL (ACON Laboratories, Inc, San Diego, CA, USA) test kits. The respective assays were reported according to the manufacturer’s instructions
The obtained data was inputted into Microsoft Excel and subsequently cleaned, prior to exporting to the software Graph Pad Prism version 9 for statistical analysis. Descriptive statistics (frequency tables and summary indices) were generated. Chisquare was employed to identify significant associations between the investigated infections and sociodemographic and behavioural risk factors. The level of significance was set at p < 0.05 with a 95% confidence interval.
Prior to obtaining informed consent, subjects were informed about the objectives of the study emphasizing confidentiality in handling their data. All authors hereby declare that the study was performed in accordance with international ethical standards.
A total of 200 consenting inmates were enrolled in the study with a mean age of 33.23. Most of them were between the age groups of 21-30 (43.5%) and 31-40 (36.5%) years. Male inmates predominated in the study with about 69% while 31% were female. They were mostly Christians (94%), single (72.5%), having completed at least secondary school education (60%) and self-employed prior to incarceration (54%). The majority of them had been incarcerated for 1-3 years (44%).
The overall prevalence of HIV in the current study was 6.5%, the prevalence of syphilis was 4.5% while HIV-syphilis co-infection was 0.5% as shown in figure 2 below
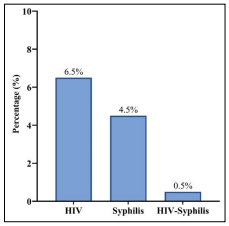
Figure 2: Overall prevalence of HIV, Syphilis and HIV-Syphilis Co-Infection among inmates
The duration of current incarceration was the only sociodemographic factor significantly associated with HIV infection (p = 0.0023). The entire prevalence of HIV recorded in the study was present among inmates that have been incarcerated for at least 1year, with a prevalence of 25% reported among inmates incarcerated for 7 or more years. The age, sex, marital status, level of education, occupation, religion, and previous incarceration were not significantly associated with HIV infection (p > 0.05) as shown in table 1
A history of blood oath was significantly associated with HIV infection (p = 0.0112), with a prevalence of 17.2% reported among inmates with a history of blood oath. Inmates who shared personal sharp objects with other inmates had a significantly higher prevalence of 12.2% than those who do not share. A significant association was also found between a history of transactional sex and HIV infection (p = 0.0236), a higher prevalence of HIV infection (12.1%) was reported among those with a history of transactional sex in the current study as shown in table 2.
There were no significant associations between the sociodemographic details of the inmates and the prevalence of syphilis (p > 0.05) as evident in table 3.
Intravenous drug use was significantly associated with syphilis infection (p = 0.0217), with a prevalence of 8.5% reported among inmates who use intravenous drugs. Inmates who share personal belongings with other inmates had a significantly higher prevalence of 8.5% than those who do not share. A significant association was also found between homosexuality and syphilis infection (p = 0.0217), and a higher prevalence of HIV infection (13%) was reported among those involved in homosexuality in the current study as shown in table 4.
Table 1: Prevalence of HIV and Sociodemographic Risk Factors of Inmates
| Variable | Positive(%) | Negative(%) | Total(%) | χ2 | Df | p-value |
| Age | ||||||
| ≤ 20 | 0 (0) | 7 (100) | 7 (100) | 2.7 | 4 | 0.6107 |
| 21 – 30 | 5 (5.7) | 82 (94.3) | 87 (100) | |||
| 31 – 40 | 7 (9.6) | 66 (90.4) | 73 (100) | |||
| 41 – 50 | 1 (5) | 19 (95) | 20 (100) | |||
| ≥ 51 | 0 (0) | 13 (100) | 13 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Sex | ||||||
| Male | 9 (6.5) | 129 (93.5) | 138 (100) | 0.00035 | 1 | 0.9852 |
| Female | 4 (6.5) | 58 (93.5) | 62 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Marital Status | ||||||
| Single | 10 (6.9) | 135 (93.1) | 145 (100) | 1.2 | 3 | 0.7439 |
| Married | 3 (5.9) | 48 (94.1) | 51 (100) | |||
| Widowed | 0 (0) | 3 (100) | 3 (100) | |||
| Separated/Divorced | 0 (0) | 1 (100) | 1 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Level of Education | ||||||
| No formal education | 0 (0) | 2 (100) | 2 (100) | 0.61 | 3 | 0.8932 |
| Primary | 2 (4.7) | 41 (95.3) | 43 (100) | |||
| Secondary | 9 (7.5) | 111 (92.5) | 120 (100) | |||
| Tertiary | 2 (5.7) | 33 (94.3) | 35 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Occupation | ||||||
| Student | 0 (0) | 15 (100) | 15 (100) | 1.2 | 4 | 0.8774 |
| Unemployed | 1 (7.1) | 13 (92.9) | 14 (100) | |||
| Self-Employed | 8 (7.4) | 100 (92.6) | 108 (100) | |||
| Private Employment | 3 (6.3) | 45 (93.8) | 48 (100) | |||
| Civil servant | 1 (6.7) | 14 (93.3) | 15 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Religion | ||||||
| African Tradition | 0 (0) | 3 (100) | 3 (100) | 0.76 | 3 | 0.8599 |
| Christian | 12 (6.4) | 176 (93.6) | 188 (100) | |||
| Muslim | 1 (12.5) | 7 (87.5) | 8 (100) | |||
| Judaism | 0 (0) | 1 (100) | 1 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Previous Incarceration | ||||||
| Yes | 3 (11.5) | 23 (88.5) | 26 (100) | 1.2 | 1 | 0.2639 |
| No | 10 (5.7) | 164 (94.3) | 174 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Duration of Current Incarceration | ||||||
| < 1 | 0 (0) | 45 (100) | 45 (100) | 14 | 3 | 0.0023* |
| 1 - 3 | 5 (6) | 79 (94) | 84 (100) | |||
| 4 - 6 | 3 (5.9) | 48 (94.1) | 51 (100) | |||
| ≥ 7 | 5 (25) | 15 (75) | 20 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
Table 2: Prevalence of HIV and Behavioural Risk Factors of Inmates
| Variable | Positive (%) | Negative (%) | Total (%) | 2, Df | OR 95% CI | p-value |
| History of alcohol use | ||||||
| Yes | 10 (6.9) | 135 (93.1) | 145 (100) | 0.14, 1 | 1.3 0.35 - 4.5 | 0.7119 |
| No | 3 (5.5) | 52 (94.5) | 55 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever had a blood oath | ||||||
| Yes | 5 (17.2) | 24 (82.8) | 29 (100) | 6.4, 1 | 4.2 0.08 - 0.70 | 0.0112* |
| No | 8 (4.7) | 163 (95.3) | 171 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Shave hair locally | ||||||
| Yes | 11 (7.8) | 130 (92.2) | 141 (100) | 1.3, 1 | 2.4 0.55 - 11 | 0.2484 |
| No | 2 (3.4) | 57 (96.6) | 59 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever had a body piercing | ||||||
| Yes | 4 (6) | 63 (94) | 67 (100) | 0.047, 1 | 0.87 0.29 - 2.9 | 0.8292 |
| No | 9 (6.8) | 124 (93.2) | 133 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever had a blood transfusion | ||||||
| Yes | 2 (7.4) | 25 (92.6) | 27 (100) | 0.042, 1 | 1.2 0.25 - 4.9 | 0.8371 |
| No | 11 (6.4) | 162 (93.6) | 173 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever been tattooed | ||||||
| Yes | 3 (4.3) | 66 (95.7) | 69 (100) | 0.80, 1 | 0.55 0.16 - 2.0 | 0.3702 |
| No | 10 (7.6) | 121 (92.4) | 131 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Intravenous drug use | ||||||
| Yes | 4 (4.9) | 78 (95.1) | 82 (100) | 0.60, 1 | 0.62 0.21 - 2.1 | 0.438 |
| No | 9 (7.6) | 109 (92.4) | 118 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| History of illicit drug use | ||||||
| Yes | 3 (3.1) | 94 (96.9) | 97 (100) | 3.6, 1 | 0.30 0.086 - 1.1 | 0.0579 |
| No | 10 (9.7) | 93 (90.3) | 103 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| History of surgery | ||||||
| Yes | 1 (3.7) | 26 (96.3) | 27 (100) | 0.4016, 1 | 0.5160 0.046 - 3.1 | 0.9506 |
| No | 12 (6.9) | 161 (93.1) | 173 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Share personal belongings with other inmates | ||||||
| Yes | 9 (7.3) | 114 (92.7) | 123 (100) | 0.35, 1 | 1.4 0.43 - 4.4 | 0.5536 |
| No | 4 (5.2) | 73 (94.8) | 77 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Share personal sharp objects with other inmates | ||||||
| Yes | 9 (12.2) | 65 (87.8) | 74 (100) | 6.2, 1 | 4.2 1.3 - 13 | 0.0128* |
| No | 4 (3.2) | 122 (96.8) | 126 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Multiple sex partners | ||||||
| Yes | 9 (6.7) | 126 (93.3) | 135 (100) | 0.019, 1 | 1.1 0.32 - 3.3 | 0.8904 |
| No | 4 (6.2) | 61 (93.8) | 65 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Sex with non-marital partners | ||||||
| Yes | 8 (6.2) | 121 (93.8) | 129 (100) | 0.053, 1 | 0.87 0.26 - 2.4 | 0.8175 |
| No | 5 (7) | 66 (93) | 71 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Condom use during extra-marital sex | ||||||
| Yes | 11 (8.7) | 116 (91.3) | 127 (100) | 2.7, 1 | 3.4 0.78 - 16 | 0.102 |
| No | 2 (2.7) | 71 (97.3) | 73 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Number of lifetime sexual partners | ||||||
| 1 - 5 | 6 (6.5) | 87 (93.5) | 93 (100) | 2.2, 4 | - | 0.7079 |
| 6 - 10 | 4 (9.8) | 37 (90.2) | 41 (100) | |||
| 11 - 15 | 1 (5.6) | 17 (94.4) | 18 (100) | |||
| 16 - 20 | 0 (0) | 20 (100) | 20 (100) | |||
| ≥ 21 | 2 (7.1) | 26 (92.9) | 28 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever engaged in homosexuality | ||||||
| Yes | 2 (8.3) | 22 (91.7) | 24 (100) | 0.15, 1 | 1.4 0.29 - 5.4 | 0.6977 |
| No | 11 (6.3) | 165 (93.8) | 176 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| Ever had transactional sex | ||||||
| Yes | 8 (12.1) | 58 (87.9) | 66 (100) | 5.1, 1 | 3.6 1.1 - 9.9 | 0.0236* |
| No | 5 (3.7) | 129 (96.3) | 134 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
| History of STI | ||||||
| Yes | 5 (6.3) | 75 (93.8) | 80 (100) | 0.014, 1 | 0.93 0.33 - 3.1 | 0.9068 |
| No | 8 (6.7) | 112 (93.3) | 120 (100) | |||
| Total | 13 (6.5) | 187 (93.5) | 200 (100) | |||
* Statistical significance p < 0.05
Table 3: Prevalence of Syphilis and Sociodemographic Risk Factors
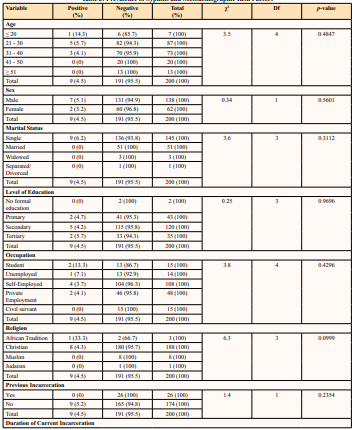
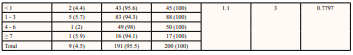
* Statistical significance p < 0.05
Table 4: Prevalence of Syphilis and Behavioural Risk Factors of Inmates
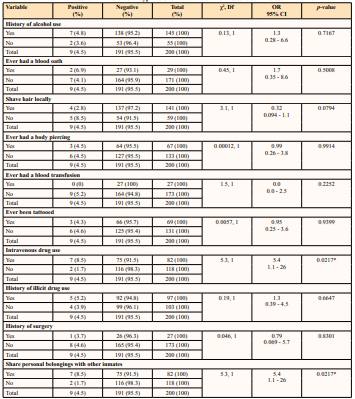
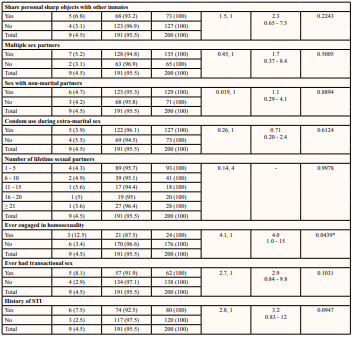
* Statistical significance p < 0.05
The seroprevalence of HIV reported in this study was 6.5%. This is attributable to the fact that there is an increased likelihood of engaging in risky behaviours such as sharing of needles and unprotected sexual activity among incarcerated individuals. The prison environment can be stressful and may lead to increased drug use and risky sexual behaviours. Additionally, prisoners may have limited access to HIV prevention and treatment services, which can further contribute to the spread of the virus. This report is comparable with the study in the Lagos prison (9%) but varies from studies undertaken in other correctional centres, such as the 1% found in inmates in Sokoto [35,36], and lower than the findings by Jeremiah et al. [22]. The finding from this study is lesser than the reported prevalence of 18% in Nasarawa State prison [37], the 12% obtained in Kaduna prison and also lesser than the prevalence found in Ghana (19.2%) [38,39].
In this study, a significant association was found between the prevalence of HIV infection and the duration of current incarceration (p = 0.0023). The entire seroprevalence observed in this study was found among inmates that have been incarcerated between 1 year to more than 7 years, suggestive of infection during the incarceration. This observation is likely due to the increased likelihood of engaging in risky behaviours over time, as well as the lack of access to HIV prevention and treatment services that can occur during prolonged periods of incarceration. A study among inmates in Iran corroborates the findings of this study by showing a correlation between the duration of incarceration and HIV infection [40].
There was a significant association between HIV infection and a history of blood oath (p = 0.0112). An HIV prevalence of 17.2% was observed among inmates with a history of blood oath with an odds ratio of 4.2. Blood oath-taking involves the exchange of blood between individuals, typically as a sign of loyalty or solidarity. This practice can increase the risk of HIV transmission if one or both of the individuals involved are HIV-positive, as the virus can be present in the blood and easily transmitted through the exchange. In the prison environment, where close relationships and alliances may form between prisoners, blood oath-taking can be more common, particularly in gangs or other groups. This can increase the risk of HIV transmission among prisoners, as well as the potential for HIV to spread beyond the prison environment if released prisoners continue the practice in their communities. This observation is comparable with a study by Dan-Nwafor et al. [41], who reported a higher prevalence of a bloodborne viral infection among inmates with a history of taking blood oath; while the cited study was limited to Hepatitis B, it is worthy to note that HIV and hepatitis B are viral infections easily transmitted via unsterilized sharp objects which is the case in a good number of these blood oaths. It can as such be argued that a similar trend is obtainable with the prevalence of HIV in the current study. Studies among populations other than the incarcerated have associated the incidence and prevalence of HIV infection with practices related to blood oaths [42].
Transactional sex as a risk factor was significantly associated with the prevalence of HIV infection in this study (p = 0.0236). A prevalence of 12.1% was recorded among inmates who had engaged in transactional sex with an odds ratio of 3.6. These inmates might acquire this infection from their lifestyles before incarceration such as prostitution, patronising commercial sex workers or during incarceration where other inmates provide materials such as food, clothes, and protection in exchange for sex [43]. In the prison environment, where sexual activity may be more secretive and limited, some prisoners may engage in transactional sex as a means of obtaining drugs, money, or other resources. This can increase the risk of HIV transmission if condoms are not used or if one of the partners is already HIV-positive. Additionally, the power dynamics within a prison can make it difficult for prisoners to negotiate safe sex practices, further increasing the risk of transmission. Previous studies have also identified commercial sex workers and transactional sex as risk factors in prisons [44, 45]. In prisons where basic hygienic materials are rarely provided, inmates resort to sharing these materials especially sharp objects such as razor blades, shaving sticks, clippers, nail cutters, etc. The current study identified the sharing of personal objects with other inmates as a risk factor and it is significantly associated with the transmission of HIV infection (OR = 4.2, p = 0.0128). According to this study, 12.2% of inmates who share sharp objects were positive for HIV infection. While the risk of transmission through these objects is lower than that of sharing needles, it is still possible to transmit HIV if these items are contaminated with infected blood. This risk is particularly relevant in the prison environment, where access to personal hygiene items may be limited and sharing of these items may be more common. The report in the current study is corroborated by similar studies by researchers in developed and developing countries [44 - 47].
The seroprevalence of syphilis in the current study was observed to be 4.5%, this is similar to the prevalence of 6% in North central Nigeria [27]. The reported prevalence in the current study is, however, lower than those reported in a Pakistani prison (8.9%) [48], and 12.8% reported in a Colombian prison [49]. One of the key factors attributable to the findings in the current study is the high prevalence of risky sexual behaviours, such as unprotected sexual contact and sex with multiple partners, which are common in prison settings. In addition, drug use and sharing of injection equipment in prisons can also contribute to the spread of syphilis and other blood-borne infections. Limited access to healthcare services, including testing and treatment for syphilis, is also a contributing factor, as many prisons lack adequate resources for disease prevention and management. Finally, the prison environment, including overcrowding and poor sanitation, can facilitate the spread of syphilis and other infectious diseases.
Sociodemographic factors such as age, sex, and marital status were not significantly associated with the prevalence of syphilis in the current study. On the contrary, a Pakistani study associated marital status with the prevalence of syphilis [48]. Marital status may be associated with syphilis infection among prisoners, although the evidence on this association is limited. It has been suggested that prisoners who are married or in a stable relationship may have a lower risk of syphilis compared to those who are single or have multiple sexual partners. This may be because prisoners in committed relationships may be less likely to engage in highrisk sexual behaviours, such as unprotected sexual contact and sex with multiple partners. However, other studies have not found a significant association between marital status and syphilis infection among prisoners [50], and more research is needed to fully understand the relationship between these factors. Several studies have shown that certain sociodemographic factors, such as age, gender, and race/ethnicity, can increase the risk of syphilis infection among prisoners. For example, younger prisoners may be at a higher risk of syphilis [51], due to engaging in more highrisk behaviours, such as unprotected sexual contact and drug use. Male prisoners have also been found to have a higher prevalence of syphilis compared to female prisoners [48], which may be due to higher rates of sexual contact with other male prisoners, some other studies did not find such an association [50]. Other factors, such as education level and socioeconomic status, may also play a role in the risk of syphilis infection among prisoners.
It was discovered that intravenous drug use was a significant risk factor in the spread of syphilis (p = 0.0217) with a prevalence of 8.5% reported among inmates involved in the use of intravenous drugs. Intravenous drug use, particularly the sharing of injection equipment such as needles and syringes, can increase the risk of blood-borne infections such as syphilis [52]. In prisons, where drug use is common and access to clean injection equipment may be limited, the risk of syphilis infection through intravenous drug use is particularly high. In addition, some prisoners may use drugs as a coping mechanism [53], to deal with the stress and isolation of the prison environment, which can further contribute to the spread of syphilis and other infections. Sharing unsterilized needles and syringes with inmates with secondary syphilis can expose an inmate to this infection. Injecting drug used was also observed as a risk factor in an Iranian prison by Nokhodian et al. [54].
Sharing of personal belongings (p = 0.0217) was also significant in the spread of syphilis. While there is limited evidence to suggest that sharing of personal belongings can be associated with syphilis infection among prisoners, it is generally considered to be a less significant risk factor compared to other transmission routes such as sexual contact and intravenous drug use. Syphilis is primarily spread through direct contact with infected skin or mucous membranes, particularly during sexual activity, and it is not considered to be highly contagious through casual contact or sharing of personal belongings. However, in some cases, sharing personal items such as toothbrushes, and other grooming tools may be associated with a risk of transmission if contaminated blood is present on the item [55, 56].
Engagement in homosexuality (p = 0.0439) was observed as a risk factor in the distribution of syphilis infection. In prisons, where same-sex sexual behaviour may be more common due to the lack of access to opposite-sex partners [57], male-to-male sexual contact is a significant risk factor for syphilis transmission [58]. Studies have consistently shown that male prisoners who engage in same-sex sexual behaviour have a higher prevalence of syphilis compared to those who do not [59]. This is likely due to the higher risk of direct contact with infected skin or mucous membranes during anal sex, which can facilitate the spread of syphilis and other sexually transmitted infections. Engagement in oral sex is common among homosexuals and this is a direct transmission route for Treponema pallidum. Studies carried out by Lacey et al. [60], in North Manchester General Hospital observed an increase in syphilis infection among homosexuals.
In this study, only one case of HIV-syphilis infection was recorded with a prevalence rate of 0.5%. Since HIV and syphilis are sexually transmitted infections, this coinfection although low is predictable, particularly as the risk factors for acquiring these infections often overlap. These risk factors can include engaging in unprotected sexual activity, sharing needles during drug use, and engaging in transactional sex. Additionally, both HIV and syphilis can be asymptomatic for long periods, which can lead to undiagnosed and untreated infections. In the prison environment, where access to healthcare may be limited and the prevalence of these risk factors may be higher, co-infection with HIV and syphilis can be more common. Similar studies have been carried out in North central Nigeria with a prevalence rate of 3.3% [27], while Nnoruka and Ezeoke recorded a prevalence rate of 2.1% of HIV-syphilis infection in Enugu State, Nigeria [61]. Studies have also recorded very little to no prevalence of HIV-syphilis co-infection. A prevalence of 0% was reported by Nokhodian et al. [54], Azarkar et al. [62], as well as in the study by Ghanbarzadeh et al. [63]. The prevalence of 0.9% reported in a Brazilian prison [64], corroborates the findings of the current study. HIV infection may modify the primary stage of syphilis, leading to an atypical presentation and/or multiple ulcers that may be with genital herpes and lesions suiting the picture of painless desolate ulcers with indurations. These signs are experienced in 31% of patients with syphilis [65].
The study highlights a high prevalence of HIV and syphilis among incarcerated individuals in Port Harcourt, co-infections of HIV and syphilis are considerably low among this very high-risk group in Port Harcourt, Nigeria. The duration of incarceration, history of blood oath, transactional sex and sharing of personal belongings were found to be significantly associated with the prevalence of HIV while intravenous drug use, sharing of personal belongings and homosexuality were significantly associated with the prevalence of syphilis in the studied population. The findings of this study necessitate further research on the implementation of interventions targeting specific high-risk populations like prisoners. The relatively high prevalence of HIV and syphilis reported in this study underlines the need for a multipronged approach towards HIV and syphilis prevention and treatment among the incarcerated in prisons in the country. Reducing the prevalence of HIV and syphilis in prisons requires a comprehensive approach that addresses the underlying risk factors for these infections. Access to HIV and syphilis testing and treatment should be provided to all prisoners, regardless of their risk level as this can help to identify and treat infections early, reducing the risk of transmission and improving health outcomes. Employing stringent means to ensure unsafe and non-consensual sexual practices are mitigated should be prioritised in the prison facility in addition to developing effective measures against the proliferation of intravenous drug use among inmates. Sterile injection equipment for legitimate medical procedures should also be made available and handled by healthcare workers in the prison. Access to personal hygiene items, such as razors and toothbrushes, should be provided to all prisoners to reduce the risk of blood-borne infections. Sanitation measures, such as regular cleaning of common areas and equipment, can also help to reduce the spread of infections.
The authors thank the inmates who participated in this study. We also appreciate the support received from the staff and management of the Maximum Security Custodial Centre, Port Harcourt as well as the Department of Medical Laboratory Science, Rivers State University, Port Harcourt.
This was an investigator-initiated study. The research did not receive any specific grant from funding agencies in the public, private or not-for-profit sectors. The authors served as sponsors for the study.
All authors listed have made a direct, substantial and intellectual contribution to the study in addition to reviewing and approving the manuscript for publication.
The authors have declared that no competing interests exist.
