Author(s):
Aims: The current study aimed to determine the effects of various fatty acids on the expression of glucose-6-phos- phatase-α (G6Pase-α) and pyruvate dehydrogenase kinase 4 (PDK4).
Methods: HepG2 cells were seeded in a 96-well plate with Dulbecco’s Modified Eagle Media, high glucose and bovine serum albumin with 25 μmoll of each fatty acid individually (butyric, politic, stearic, oleic, linoleic, lin- olenic, and arachidonic acids) for 24 hours at 37°C with and without 10 minute incubation of 100 nmol insulin. qPCR was performed using G6Pase-α and PDK4 primers; fold-changes in gene activity were determined.
Results: Butyric (-2.0) and linoleic (-65.8) acids caused down regulations of G6Pase-α in the presence of insulin while linoleic (-96.6) acid caused a down regulation of PDK4 in the presence of insulin; all caused upregula- tions of both these genes in the absence of insulin. Conclusions: This study suggests that linolenic acid, which is present in soybeans, walnuts, and kiwi seeds, is beneficial to processing glucose and could increase insulin sensitivity through molecular influence in patients with metabolic syndrome or serve as prevention. Other fatty acids tested, with exception of butyric acid, did not show beneficial effects in the direction of decreasing glucose.
It has been reported that 23% of adults in the U.S. have metabolic syndrome [1]. Although this is generally a combined risk of high blood pressure along with high serum glucose and cholesterol levels, metabolic syndrome is best explained by insulin resistance with evidence indicating that it begins with excess central adiposity [2]. While it has been established that insulin resistance can be improved by weight loss from physical activity and dietary control, the effect of various foods on insulin resistance, and more specifically, cellular mechanisms involved in glucose metabolism have come into question. Dyslipidemias are problematic in the U.S. with around 50% of adults having some form of lipid abnormality [3]. there is evidence that free fatty acids are a link between obesity and insulin resistance [4]. Most obese subjects have elevated free fatty acid levels and commonly these elevations inhibit glucose uptake stimulated by insulin. However, a certain level of lipids in the diet are necessary for healthy living, with some
fatty acids specifically being beneficial. For example, it is known that omega-3 fatty acids enhance insulin sensitivity through repressing macrophage-induced tissue inflammation [5].
Butyric acid is a short chain saturated fatty acid found
in butter, cow and human milk, and is a product of
intestinal fermentation of fiber. This fatty acid of ratio
4:0 has been found to be beneficial to those with irritable
bowel disease as well as those with metabolic syndrome
[6-7]. In the presence of a high fiber diet, short chain
fatty acids such as butyric acid can be produced by
bacteria in the colon and result in increases in glucagonlike peptide-1 and improved regulation of glucose levels
[8]. Butyric acid has also been shown to increase gene
expression of GLUT2 and GLUT4 as well as IRS1 in
human hepatocytes and preadipocytes, respectively,
indicating that it can increase insulin sensitivity by
directly affecting the insulin pathway [9-10].
Palmitic acid is a saturated fatty acid with a carbon
to double bond ratio of 16:0. The World Health
Organization stated that evidence is present that this
fatty acid increases low-density lipoprotein (LDL) levels
and, thus, the risk of cardiovascular disease [11]. A study
involving a population in Costa Rica indicated that a diet
rich in palm oil through cooking, which contains high
levels of palmitic acid, was associated with decreased
high-density lipoprotein (HDL) and a 2.5-fold increase
in acute myocardial infarction when compared to those
on high vegetable and fruit diets [12].
Stearic acid, derived naturally from soybeans, coconut,
and sunflowers, is a saturated fatty acid with ratio of
18:0. One study involving a literature review evaluating
the effects of stearic acid concluded that it was associated
with lower LDL in comparison to other saturated fats,
but was raised LDL in comparison to unsaturated fatty
acids [13]. A different study suggested that stearic acid
is associated with insulin resistance because those with
higher levels of serum stearic acid were associated with
increased C-peptide levels [14].
Oleic acid is an 18:1 monounsaturated fatty acid that
is natural in vegetable and animal oils and fats. Several
studies have shown the health benefits of oleic acid,
including the reduction of LDL and total cholesterol due
to daily intake of skim milk supplemented with oleic acid,
polyunsaturated fatty acids, folic acid, and B vitamins
[15]. Thus, food technology that reduces saturated fats
with the introduction of monounsaturated, such as
oleic acid, or polyunsaturated fats can boast decreases
in cardiovascular disease risk [16].
Linoleic acid is an 18:2 polyunsaturated omega-6 fatty
acid and can be found in various oils, seeds, and egg
yolk. Some conjugated forms of linoleic acid (CLA),
especially 10,12-CLA, have been shown to reduce
adiposity in animal and some human models as well as
cause anti-inflammatory effects [17-18]. While some
studies of the effect of linoleic acid on insulin regulation
have been inconclusive, it has been suggested that it
may take long-term exposure for beneficial effects to
take place [19].
The health benefits of linolenic acid, an 18:3
polyunsaturated fatty acid found in vegetable oils, are
somewhat established. Supplementation of α-linolenic
acid was shown to improve serum adiponectin levels
and insulin sensitivity through decreases in hemoglobin
A1C and fasting plasma glucose levels in those with
diabetes mellitus type 2 [20]. In diabetic rats this fatty
acid was shown to produce cardioprotection through
anti-inflammatory and anti-oxidative stress [21].
Conversely, low levels of both linoleic and linolenic
acids were found to be associated with chronic kidney
disease in patients with diabetes mellitus type 2 [22].
Arachidonic acid is a 20:4 polyunsaturated omega-6 fatty
acid found in meat and eggs and it plays a complex role
in repairing skeletal muscle tissue and is important in
neurological health. Its metabolites initiate and resolve
inflammation and have been linked to cardiovascular
disease and obesity [23].
It has been reported that the mechanism through which fatty acids inhibit insulin activation in muscle tissue is by inhibiting insulin receptor subtrate-1-associated phosphatidyl inositol 3-kinase activity [24]. This occurs through increases in intracellular diacylglycerol and fatty acyl-CoA and resulting increases in protein kinase C-o, which causes increased phosphorylation of IRS-1 Ser-307 and tyrosine phosphorylation, all resulting in decreased glucose transport by insulin influence. Theeffect of individual fatty acids on genes that are involved in glucose transport is also of interest to determine their effects separately
There are numerous genes involved in mechanisms in the insulin pathway. For example, glucose-6- phosphatase-α (G6Pase-α) is expressed in tissues where gluconeogenesis occurs, such as the muscles, liver, and kidneys [25]. This enzyme forms a complex with the glucose-6-phosphatase transporter to maintain homeostasis of serum glucose levels between meals. The activity of insulin decreases G6Pase-α which prevents additional glucose from entering circulation. Those who are insulin resistant do no have the protection of insulin that prevents gluconeogenesis and, thus, the glucose levels continue to rise. If this gene is upregulated, glucose is expected to rise.Pyruvate dehydrogenase complex (PDC) is suppressed by pyruvate dehydrogenase kinase 4 (PDK4), which is present in the liver, heart and kidneys [26]. This is important for synthesizing glucose when it is needed. Unnecessary suppression of this complex by enzymes such as PDK4 likely promotes metabolic syndrome. That is, increases in PDK4 would lead to increases in glucose levels, glycosylated hemoglobin, adiposity, and metabolic syndrome.
The current research set out to determine the effect of various fatty acids on activities of G6Pase-α and PDK4, both in the presence and absence of insulin. It was hypothesized that butyric acid would have a downregulation of the activities of these genes since it has been shown to have a positive effect in those with metabolic syndrome by improving regulation of glucose [6-8]. It was further hypothesized that others would result in decreases in activities in these genes to varying degrees, potentially based on saturation degree and hydrocarbon length.
HepG2 (ATCC® HB-8065) were grown in Eagles Minimum Essential Medium (ATCC® 30-2003) with 10% fetal bovine serum (ATCC® 30-2020) and 1% penicillin-streptomycin (Gibco™ 15140148) [27-28]. 1.25 x 105 cells per well were seeded into a 96 well plate with 100 μl of Dulbecco’s Modified Eagle Media, high glucose (Gibco™ 11995065) and 1.2 mg/ml of bovine serum albumin (Fisher™ BP671) [28-29]. Fatty acidsbutyric, palmitic, stearic, oleic, linoleic, linolenic, and arachidonic- (Sigma® W222100, 10931, 43051, S4751, O1008, S1376, L2376, 10931) were added to separate wells at 25 μmol with and without 100 nmol of insulin, in triplicate along with controls without fatty acids [30]. After 24 hours of incubation at 37°C, 100 nmol of insulin was added to one set of the fatty acids and a control for 10 minutes incubation. To another set of fatty acids and a control, no insulin was added. RNA was extracted using RNeasy Mini Kit (Qiagen® 74104) and qPCR was performed using G6Pase-α and PDK4 primers (Qiagen® 330001) [31]. Fold changes of gene activity for each fatty acid compared to controls were determined using 2(-δδCt). Results less than 1 were divided into -1 and results greater than 1 were divided by 1.
The greatest fold-change in gene activity of G6Pase-α resulted from linolenic acid in the presence of insulin with a downregulation of 65.8 (Table 1). The greatest fold-change in gene activity of PDK4 resulted from linolenic acid in the presence of insulin with a downregulation of 96.6. All other fatty acids resulted in upregulations of activities of both genes with the exception of butyric and stearic acids causing a downregulation of G6Pase-α in the presence of insulin, although the fold-change of -1.4 is not considered significant (Figures 1 and 2). The greatest upregulation of G6Pase-α in the presence of insulin was caused by arachidonic at 27.7, while the greatest increase in activity of this gene in the absence of insulin was caused by palmitic acid at 90.6. For PDK4, the greatest upregulation in the presence of insulin (142.6) occurred due to arachidonic acid and in the absence of insulin was caused by linolenic acid at 624.4.
| FA: | G6Pase-α, Ins. | G6Pase-α, No Ins. | PDK4, Ins. | PDK4, No Ins |
|---|---|---|---|---|
| Butyric | -2.0 | 21.1 | 2.0 | 236.8 |
| Palmitic | 9.2 | 90.6 | 15.5 | 403.6 |
| Stearic | -1.4 | 2.6 | 1.9 | 12.5 |
| Oleic | 15.2 | 19.5 | 35.0 | 292.2 |
| Linoleic | 25.32 | 67.9 | 123.3 | 461.2 |
| Linolenic | -65.8 | 45.7 | -96.6 | 624.4 |
| Arachidonic | 27.7 | 35.9 | 142.6 | 434.2 |
Table 1: Fold change in gene activity for each FA, with and without the presence of insulin. Ins.= insulin; FA= fatty acid.
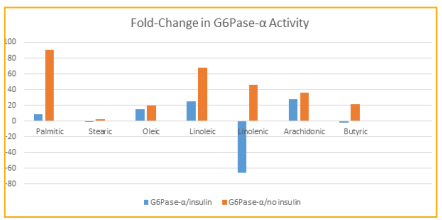
Figure 1: Fold-change in G6Pase-α activity for each FA, with and without the presence of insulin.
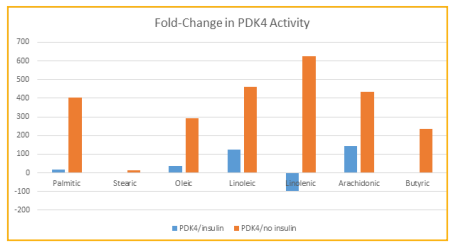
Figure 2: Fold-change in PDK4 activity for each FA, with and without the presence of insulin.
Fold-changes of greater than 2.0 or less than -2.0 are considered significant. Most effects of fatty acids in this study were detrimental according to the changes in gene activities. activity was increased in the presence of insulin when exposed individually to palmitic, oleic, linoleic, and arachidonic acids. Without the presence of insulin, all fatty acids caused increases in activity. Only butyric and linolenic, in the presence of insulin, caused a downregulation in the activity of this gene, which indicates that these fatty acids may have the effect of decreasing glucose levels.Similarly, for PDK4 only linolenic acid, in the presence of insulin, and no fatty acids in the absence of insulin, was downregulated indicating the same likely impact by this fatty acid on glucose levels as well. In this study the majority of the individual fatty acids had a detrimental effect on the levels of 2 genes which are important in processing glucose, and would thus likely have a negative effect on metabolic disorder. The exception for both genes was linolenic acid, which caused a downregulation of both G6Pase-α and PDK4 in the presence of insulin. Linolenic acid is found in seeds and nuts such as chia sage, kiwi, flax, purslane, walnut, and soybean [32- 33]. Linolenic acid has previously been associated with improvement of serum adiponectin levels as well as improving hemoglobin A1C and fasting plasma glucose levels in those with diabetes [20]. In diabetic rats it was found to provide cardiac protection through antiinflammatory and anti-oxidative stress [21]. Perhaps the most promising recent study that coincides with the present study is one whereby a supplement of 45g of walnuts, which contain linolenic acid, given each day over a 16 week period to 84 Korean adults improved the status of those with metabolic syndrome by increasing HDL and adiponectin levels, decreasing fasting glucose levels, and improving hemoglobin A1C levels [34]. Butyric acid has also been previously shown to have beneficial effects on subjects with type 2 diabetes mellitus [7]. It has been shown to decrease glucose levels and increase glucagon-like peptide-1 secretion to improve insulin resistance. It has also been shown to increase expression of GLUT and IRS1 in human hepatocytes and preadipocytes in vitro [9-10]. Thus, the decrease in G6Pase-α activity found in the current study by butyric acid coincides with previous findings. More studies on the effects of linolenic acid on various genes that are involved in glucose metabolism may shed more light into the overall effects this fatty acid has on metabolic disorder. In addition, rodent studies involving diets high or low in various fatty acids will allow a greater understanding of the impact they have on overall health by testing glucose, hemoglobin A1C, cholesterol and other parameters.
I would like to acknowledge the Clinical Laboratory Science Department of Winston-Salem State University as well as the funding providing by MARC U*STAR Program T34GM070416, NIGMS-RISE,
R25GM113774, and WSSU PDC Grant. I would like to thank Dr. Segun Ariwodola, PhD, for his assistance during this project.
