Author(s): Daina M Chase and Vincent S Gallicchio*
FMS-like tyrosine kinase 3 (FLT3) is important in the normal development of stem cells and the immune
system. In acute myeloid leukemia (AML), there is an activating mutation of this tyrosine kinase gene. This
mutation results in the survival and proliferation of leukemic blasts, which can result in an adverse prognosis.
Consequently, FLT3- inhibition has become of interest for the treatment of myeloid leukemias such as AML.
Tryosine kinase inhibitors (TKIs) are being developed and investigated for FLT3-mutated AML, and many are
beginning to show efficient results. AML has a progressive low survival rate with the standard medical care
being chemotherapy. AML patients have shown to have a mutation in the FLT3 gene in more than 30% of
patient cases. Knowing this correlation, it is important to further investigate tyrosine kinase inhibitors use for
treatment. This review summarizes information of what FMS-like tyrosine kinase 3 is and how it is important
in the process of hematopoiesis. Then it will discuss the correlation of FLT3 mutation in patients with AML.
Lastly, the advancement of treatment of AML using FLT3 inhibitors or TKIs will be discussed.
The process by which hematopoietic stem cells and
progenitors differentiate into blood cells of various
lineages is called hematopoiesis. This process involves
many interactions of transcription factors that influence
the expression of downstream genes and they also
facilitate differentiation and proliferation signals [1].
Cytokines also have a role of maintenance, expansion, and
differentiation of hematopoietic stem cells in the bone
marrow. The bone marrow comprises of hematopoietic
stem cells and provides a microenvironment for them
known as the stroma. This is where they undergo selfrenewing and self-divisions of their stem cell pool.
This will provide them an environment where they
can differentiate into the mature cells they need to be
to achieve their physiological function. During this
process, cytokines help contribute to this differentiation
of the cells [2]. Therefore, cytokines are important
regulations in hematopoietic development. They
provide a key role in the transfer of signaling to cells
which affect their survival, proliferation, differentiation,
and maturation. FMS-like tyrosine kinase (FLT3) is a
typical example of a hematopoietic cytokine. However,
this cytokine is often overexpressed in leukemias or it is
mutated [3].
Accordingly, hematopoiesis can involve mutations in
the regulatory genes that that are capable of promoting
leukemogenesis [1]. Fms-like tyrosine kinase 3 has a
critical role in the normal development of stem cells
and the immune system [4]. FLT3 gene is the gene
that encodes this tyrosine kinase receptor that controls
survival, proliferation and differentiation of HSC.
If this gene is mutated, then it causes deregulation
of the balance between cell proliferation and cell
differentiation [1]. FL is a cytokine that acts through
FLT3 and has pleiotropic and potent effects on the
development of HSCs and the immune system [2]. FLT3
is a cell surface receptor tyrosine kinase that is expressed
by immature hematopoietic cells. This receptor
collaborates with other growth factors, like cytokines,
to produce proliferation of stem cells, progenitor cells,
dendritic cells, and natural killer cells. It has been
thought that FLT3 could be used as a therapeutic target
for kinase inhibitors or other approaches for patients
with mutations of this gene. This is because mutations
of FLT3 have been commonly present in unfortunate
diagnosis such as leukemia [4]. This tyrosine kinase was
first found on a murine hematopoietic progenitor cell
(HPC) but on humans it is restricted to CD34+ bone
marrow cells which includes the hematopoietic stem
cells (HSC) [5].
When a ligand binds to FLT3, dimerization of the
receptor occurs. FLT3 then self-phosphorylates its
tyrosine residues. This is shown in Figure 1, where if
the tyrosine is not phosphorylated, they are in a closed
conformation but if they are phosphorylated, they are
in the active conformation and can proceed with the
process. From this, adapter proteins are recruited and
the Ras/MAP kinase or phosphoinositide 3 kinase
(P13k) pathway is signaled. These pathways are vital in
cell survival and growth. The ligand that binds to flt3
is important in promoting the survival and growth of
hematopoietic progenitors. These progenitors include
the ones in the myeloid and B lymphoid pathway. Since
Flt3 is shown present in the CD34/CD33+ cells, it is
shown to have influence in the formation of granulocytemacrophages (GM) by human bone marrow. Flt3 also
synergizes with other cytokines. Cytokines include
interleukins, growth factors, colony forming units and
transcription factors. Furthermore, Flt3 has been shown
to have a wide array of functions and influences on cells
belonging to the lymphoid and GM pathways [5]. FLT3
ligation provides growth-stimulation and antiapoptotic
signals [6].
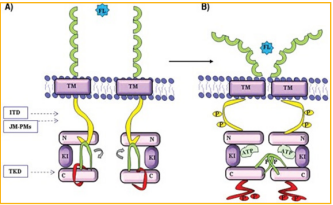
Figure 1: A. Inactive conformation of FLT3. B. Active conformation of FLT31.
In this paper, the role of FLT3 and its ligand on hematopoietic stem cells are discussed because of the interest in their clinical applications. Clinical applications include stem cell transplantation and cancer immunotherapy [2]. FLT3 is commonly mutated in acute leukemias. FLT3 internal tandem duplication mutation in acute myeloid leukemia (AML) have a poor prognosis in adult and pediatric patients. This discovered the use of FLT3 inhibitors to treat patients with acute leukemias. Many of these are still in preclinical development and some have entered clinical trials [7]. They will be discussed in this review as well as the overall importance of FLT3.
A mutation of Flt3 is commonly found in AML [2]. It is also one of the most frequent genetic alterations in AML and indicates poor prognosis [6]. Since Flt3 plays a critical role in early hematopoietic development and regulates the growth and differentiation of CD34+ hematopoietic cells, a mutation in it can lead to severe problems. It is often recommended to receive allogeneic hematopoietic stem cell transplants for patients with Flt3 mutations even though sometimes this does not work for treatment either [8]. Allogenic transplant is the transplantation of stem cells from a matching donor. The other stem cell transplantation is autologous stem cell transplant which uses stem cells directly from the patient [9]. Patients where this transplant usually does not work are those with Flt3-internal tandem duplications (ITD) due in part because they have a higher rate of relapse. ITDs are always expressed in patients but they vary in length. These duplications are in the juxta membrane domain of the Flt3 in all subtypes of AML. They result in the constitutive activation of the receptor. Researchers do not know why but they believe this is due to the active tyrosine kinase site allowing access of ATP and substrates as a result of unfolding. Since therapeutic options are limited for those with this mutation, researchers are trying to find a way to prevent this by using Flt3 inhibitors as a maintenance therapy. They believe the inhibition of this Flt3 signaling could be an effective approach of treatment [2].
A report states how wild type FLT3 (FLT3-WT) is
expressed in 93% of AML cases, almost 100% of B-cell
acute lymphoblastic leukemia (B-ALL), 87% of T-cell
acute lymphoblastic leukemia (T-ALL) and in a small
percentage of chronic myeloid leukemia [10]. These high
percentages of FLT3-WT in these diagnosis’s show how
the overexpression of FLT3 may result in the survival
and proliferation of leukemic cells. 30% of mutations in
FLT3 have been found in with AML [1].
ITD mutation results in the prevention of the association
between the JM domain and the kinase domain as can be
seen in Figure 1 for reference of where these are on the
receptor. When this mutation prevents this association,
the kinase will then undergo a conformational change
and will be constantly activated. This will therefore block
myeloid differentiation of hematopoietic progenitors
since the normal signaling pathways will be disrupted.
This report states the role of FLT3-ITD preventing
Fox03-a mediated apoptosis. This then promote the
survival and proliferation of AML cells. In addition,
FLT3-ITD and their role in increased activation of
downstream signaling which inhibits cellular phosphates
which can lead to amplification of the proliferative and
anti-apoptotic effects. Furthermore, FLT3-ITD causes
significant increase in cell proliferation since there is a
continuous activation of the receptor. This is involved in
leukemic progression. This mutation is shown in Figure
2 on the right. The figure shows the presence of the
ligand on FLT3-ITD will activate AKT phosphorylation
and increased MAPK activation. On the left, shows the
signaling pathways activation by FLT3-WT [1]. Patients
with FLT3-ITD AML tend to be younger patients that
have high white blood cell counts. These patients
have a high risk of relapse if they achieve remission.
This disease has improved in prognosis over the years
because of the advances of allogeneic hematopoietic
cell transplantation as well as FMS-like tyrosine kinase
inhibitors [11].
Studies have shown that FLT3-ITD transformed cells
injected into mice result in a leukemia-like syndrome.
These FLT3-ITD transformed cells include Ba/F3 or
32D. This study also showed that the injection showed
myeloproliferative disorder in the mice bone marrow
cells. The FLT3 mutations result in the disruption of the
kinase activity. These preclinical studies give researchers a great idea on the responsibility of FLT3 and how they
may be a viable therapeutic target for treatment of AML
[12].

Figure 2: (Left) Signaling pathway of FLT3-WT (Right) Signaling pathway of FLT3-ITD1.
Studies have shown that FLT3-ITD transformed cells injected into mice result in a leukemia-like syndrome. These FLT3-ITD transformed cells include Ba/F3 or 32D. This study also showed that the injection showed myeloproliferative disorder in the mice bone marrow cells. The FLT3 mutations result in the disruption of the kinase activity. These preclinical studies give researchers a great idea on the responsibility of FLT3 and how they may be a viable therapeutic target for treatment of AML [12].
Another study shows that FLT3 is highly expressed in mixed lineage leukemia (MLL) rearranged acute lymphoblastic leukemia [13]. Acute lymphoblastic leukemia (ALL) is in infants and is categorized by the rearrangements of the mixed lineage leukemia gene, drug resistance, and a poor treatment outcome. In this study, they show FLT3 expression in infants with MLL are higher compared to both infant and noninfant patients with ALL carrying MLL genes. The leukemic cells from infants with MLL are more sensitive to the FLT3 inhibitor than noninfant ALL cells. Their results found the use of flt3 inhibitors could be used in infants with MLL cells since they show with overexpression of FLT3. In the experiment, they assessed whether the level of expression of FLT3 was sufficient enough to self-phosphorylate and activate FLT3 in the absence of activating mutations. They examined several infant MLL and noninfant ALL patients with varying levels of FLT3 expression (Figure 3A). They also analyzed the patients expressing high levels of FLT3 were sensitive to the FLT3 Inhibitor drug, PKC412. Similarly, the patients with low level of FLT3 did not respond to the drug (Figure 3B). Furthermore, the study shows FLT3 overexpression in leukemic cells from infants with MLL rearranged ALL [13].
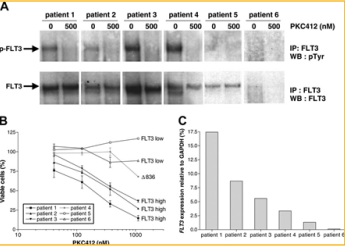
Figure 3: Observation of FLT3 phosphorylation in infant MLL patients with varying levels of FLT3 expression and noninfant ALL patients [13].
There have been a number of preclinical studies in vitro
and in vivo that have shown clinical benefits of FL, that
acts through FLT3. These are mostly done through stem
cell transplantation and cancer immunotherapy. For
stem cell transplantation, it is important to understand
how stem cells work in order to use this therapy. The
transfer of cells from the bone marrow into blood
circulation is based on the administration of cytokines.
Granulocyte colony stimulating factor (G-CSF) is an
agent used to move many stem cells (CD34+) to be
harvested and used for stem cell therapy. FL works like
G-CSF through progenitors, but FL can also mobilize
dendritic cell subsets. Since the FL can do this to the
dendritic cells, the dendritic cells show a possible
benefit of FL. The expansion of dendritic cells by FL
can possibly be used for immune recovery in early
post transplantation period. The downfall is that the
reconstitution of a diversified T cell range does not start
for at least six months and is impaired with age. This
shows that FL to be used for immune recovery may be
only used for selected patients. The interaction of these
cells is important in immunological responses especially
between DCs and NK cells. They are important in the action of anti-tumor defense to prevent the relapses
from stem cell therapy. This was shown in animal
models. This study reports how FL was used to enhance
protective immunity against pathogens in mice. They
showed this could be due the FL’s role in expanding
DCs which captures microbial antigens. They believe
this is important in reducing infections after stem
cell therapy. The introduction of FL in patients after
stem cell therapy could boost the hematopoietic and
immune recovery to enhance antitumor immunity. In
addition to stem cell therapy, there is also the studies
of cancer immunotherapy. As stated above, the DCs are
shown to induce antitumor immunity. FLs that expand
these cells are what makes them a promising tool for
treatment. This study reports a clinical trial of patients
with advanced colon and lung carcinomas. Twelve
patients were treated with a large number of autologous
DCs after administration of FL. Two out of the twelve
patients experience tumor regression and three of them
experienced the disease stabilizing [2].
A study examined the effect of FLT3 on purified human
CD34+ progenitor cells. They did this by cloning it. By
adding FLT3 to irradiated long-term marrow cultures
with CD34+ cells amplified both total and progenitor
cell production. FLT3 showed to support maintenance of
both GM and HPP progenitors in vitro. The study found
that FLT3 has an important function in the regulation
of multipotent and lineage committed hematopoietic
progenitor cells. After comparing FLT3 and SLF, they
conclude that they act similarly. FLT3 also synergizes
with a combination of cytokines to induce the ex vivo
expansion of bone marrow derived progenitor cells [14].
In one study, the expression of FLT3 within the
hematopoietic stem cell and bone marrow in mice was
investigated. They did this by using flow cytometry
and quantitative reverse transcription polymerase
chain reaction. First, they isolated the hematopoietic
stem and progenitor cells (HSPC) from the mouse
bone marrow. Since hematopoietic progenitor cells
express high levels of FLT3 at the surface, they used the
gating strategy to identify them. This part of the study
confirmed the expression of FLT3 by cells within almost
all HSPC populations, including hematopoietic stem
cells. They confirmed this finding by flow cytometry as
well but that it is most commonly found on cells with
lymphoid potential. Leukemia with FLT3-activating
mutations could have arisen from the FLT3 that is
found in the bone marrow and in the primitive stem
cell compartment. From Figure 4, it shows what this
experiment found. How HSC has receptors which
include FLT3 but as the stem cell differentiate, FLT3
is found in multiple lineages, strongly associating with
lymphoid-GM potential. It is important to understand
the expression of these receptors since it indicates the
fate of cells [5].
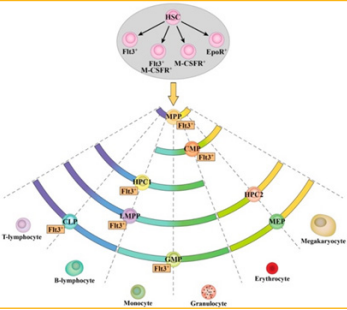
Figure 4: FLT3 expression in the murine HSPC cell line. This figure demonstrates the heterogeneity of HSPC and its expression of FLT3 [5].
In one study of the bone marrow of mice, it was shown
that mice deficient in expression of FLT3 ligand showed
deficient lymphopoiesis, preferentially affecting B cell
development. It also showed the reduction of NK and
DC. This agreed that FL could be important for the
differentiation of stem cell lineages. In addition, FLT3
and its ligand are important for stem cell maintenance.
They found that mice lacking expression of FL
demonstrated reduced numbers of early B lymphoid
progenitors. They also found in FL deficient mice, the
reduction of early T cell progenitors as well15.
The focus of one review by [16], described the function of the FLT3 receptor in the process of a therapy that
would treat T cell-mediated autoimmune diseases. First,
they explained FLT3 signaling and its role. A previous
study found the role of FLT3 in DC development was
found both in vitro and in vivo showing the treatment of
progenitor cells or mice with FLT3L led to a proliferation
of DCs. It was also shown that mice with a deficiency in
FLT3 correlated with having a deficiency in DCs. This
brought about more studies that focused on FLT3 being
used as an immunostimulatory antitumor agent and
then was found to produce anticancer effects. Since high
levels of FLT3 are shown in leukemias, more studies
showed the constitutive activation of it in leukemias
because of a mutation of FLT3. Thus, treatment with
FLT3 inhibitors has been of interest [16]. One study
showed the treatment of a FLT3 inhibitor that inhibited
type I interferon producing DCs. This in vivo treatment
of mice resulted in a phenotype that was similar to FLT3
deficient mice. However, because of implications such
as how the number of DCs returned to normal after
therapy and the lack of effect on repopulation of bone
marrow stem cells, showed more consideration needed
to be done before clinical use [17]. Next, another study
used an FLT3 inhibitor that showed similar results. They
found the decrease in DC population which decreased
an autoimmune response. There was no change in
mature B and T cells. There was, however, a decrease
in T-cell expansion. In the model that had multiple
sclerosis, it was shown after treatment that the mouse
showed significant improvement in the course of disease
[18]. This study showed how inhibiting an ongoing T
cell response via inhibition of DCs could prove to be
effective, thus treating T cell mediated autoimmune
diseases. This study supposes that targeting FLT3 would
be the best approach in treating autoimmune disease
because of its selectivity. This process would target a
signaling pathway expressed in antigen-presenting cells,
but not mature B or T cells. This process would allow
targeting of the antigen presentation which would limit
the event of targeting a wrong antigen that would be
unknown or could mutate over time. Also, since mature
B and T cells are not direct targets, this could decrease
the chance of an auto-immune response that would
destroy B and T cells [16]. As stated previously, dendritic
cells can regulate and amplify immune responses. FLT3
stimulates the production and proliferation of these
dendritic cells. In a preclinical study, mice were injected
subcutaneously with FL for 8 days. They found by using
flow cytometry, that mice treated showed an increase
in cellularity in the spleen. They also compared FL DCs
to the control DCs as stimulators of T-cell responses in
an allogeneic mixed lymphocyte reaction. They found
FL DCs were less effective stimulators than the control
shown in Figure 5. Also shown in the figure is that
the cytokines IL-2 and IFN-γ decreased in secretion
during allogeneic T-cell responses for the FL DCs.
Furthermore, after all of the data collected from this
study, it was shown that the treatment of FL on mice
reduced their ability to stimulate allogeneic T cells in
mixed lymphocyte reaction even though DC numbers
increased. This study further agrees with the others on
the impaired allo-stimulatory capacity of DCs from
bone marrow of FL treated mice. This study confirmed
the important of the decrease in allo-stimulation caused
by FL in vivo [19].
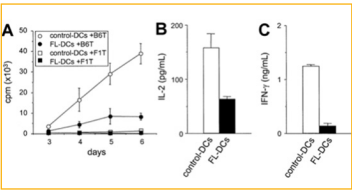
Figure 5: Comparing the data contained from control DCs and FL DC from mice [19].
Leukemic stem cells (LSC) are found in AML cells and they express high levels of myeloid cell leukemia-1 (MCL-1) even compared with normal hematopoietic cells. This suggest that AML LSCs rely on MCL-1 for survival. The AML LSCs express FLT3 and FLT3-ITD. Knowing this, this study analyzed the bone marrow of thirty adults with AML in Kyushu University Hospital. After stem and progenitor analysis of the cells in the bone marrow of these patients, they found among the thirty AML patients, that FLT3-ITD was detected in eleven of them. As shown in Figure 6, they showed the phenotypic characteristics of the bone marrow cells in normal and AML. This study showed how AML LSCs expression high levels of FLT3 compared to normal hematopoietic stem cells [6].
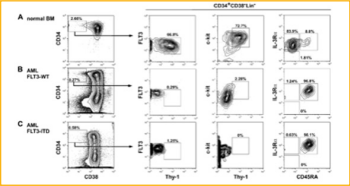
Figure 6: Phenotypic characteristics of bone marrow cells in A. (Normal bone marrow B.) AML-FTL3-Wild Type. C.) AML- FLT3-ITD [6].
A major advance in treatment of patients with FLT3
internal tandem duplication acute myeloid leukemia is
TKIs. AML is a poor prognosis and many advances are
being developed for treatment to replace the toxicity
of chemotherapy [11]. Chemotherapy with or without
allogeneic hematopoietic stem cell transplantation
has limited efficacy in AML. It only has a cure rate of
only 30-40% [20]. These advances include allogenic
hematopoietic cell transplantation as well as tyrosine
kinase inhibitor drugs. These drugs include, sorafenib,
midostaurin, gilteritnib, quizartinib etc. Sorafenib is a
multitargeted TKI. Midostraurin in 2017 was approved
by the FDA as well as gilteritnib in 2018 for clinical
use in FLT3 mutated AML [11]. Since FLT3 mutation
are found in approximately one-third of patients with
AML, FLT3 has become an attractive therapeutic target.
FLT3 inhibitors are undergoing clinical evaluations to
find the most efficient, selective, and potent compound
against FLT3 mutation [21]. The reason for this is
because of the greater potency and selectivity, the great
efficacy in FLT3- mutated acute myelogenous leukemia
(AML). This also promises less toxicity for the patient
[22]. The way these FLT3 inhibitors work is by acting
via competitive inhibition with adenosine triphosphate
on the tyrosine kinase domain. Inhibition results in
decreased autophosphorylation and its successive
activation, thus they are labelled as first generation
and second-generation inhibitors. The first generation
are not as specific for FLT3 which include tandutnib
(MLN518, CT53518), sunitnib, sorafenib, midostauin
(PKC412), and lestaurtinib (CEP701). Next, second
generation flt3 inhibiotrs are more selective and potent.
These include quizartinib (AC220), crenolanib (CP868-596), ponatinib, pacritinib (SB1518), and gliteritnib
(ASP2215). Most of these drugs are undergoing
trials in clinical settings and will be discussed below.
Furthermore, past, ongoing, and planned trials will
be discussed below for each of these inhibitors for
treatment of patients with this disease.
A study examined six different FLT3 inhibitors for
potency against mutant and wild type FLT3. They also
examined them for cytotoxic effects against a series of
primary blat samples from AML patients. They found
that the FLT3 inhibition does not always induce cell
death so some FLT3/ITD AML may not be continuously
signaling FLT3. The six FLT3 inhibitors varied in their
selectivity for FLT3 which influenced cytotoxic effect.
Some were highly selective, intermediate selective,
and less selective. From their data, they found a newly
diagnose patient with AML may respond better to the
less selective FLT3 inhibitor since their data suggest
that response rate for diagnostic specimens is higher for
them. This is shown in Figure 7, where all of the inhibitors responses are shown. It shows,
lestautnib, the less selective drugs shows a very modest
cytotoxic effect against the wild type samples whereas
sunitnib, highly selective inhibitor, shows no effect
whatsoever. They found from their data that a high
mutant allelic burden does correlated with cytotoxic
response to selective flt3 inhibition. This means that
only newly diagnosed AML patients with FLT3/ITD
would respond to FLT3 inhibition therapy [24].

Figure 7: Cytotocicity assays of FLT3ITD primary AML samples [24]
Lestauritnib is a first generation agent that is relatively nonspecific for FLT3 and usually inhibit other class III receptor tyrosine kinases such as KIT and PDGFR [23]. A phase III study in 2017 investigated newly diagnosed AML patients. They did this through a randomized assessment through combination therapy of lestaurtinib to first-line chemotherapy. Therefore, they did it through intensive chemotherapy in combination with lestaurtinib starting two days after each chemotherapy. This study showed no overall clinical benefit after the administration of lestaurtinib in combination with chemotherapy. However, they did find lower rates of relapse and improved overall survival in the patients that achieved sustained levels of FLT3 inhibitory activity. Researchers of this study acknowledge during their study that second generation of more selective FLT3 inhibitors were designed that have the apparent capability of achieving more flt3 inhibition [25].
In a preclinical study, a FLT3 inhibitor named gilteritinib
was examined. Gilteritnib is a pyrazinecarboxamide
derivative that is being studied in AML clinical
trials. This is because of its selectivity, potency, and
activity against FLT3-activating mutations. This study
compared gilteritnib to other flt3 tyrosine kinase
inhibitors (FLT3 TKIs), which include midostaurin,
sorafenib, quizartnib, and crenolanib. First, testing of
the inhibitory activity of gilteritnib against different
forms of FLT3 in leukemia cells was completed. This is
summarized in Figure 8. It was shown that gilteritnib
had similar levels of inhibition against mutations that
sorafenib and quizartinib did. In addition, from this
figure shows that gilteritnib may have the ability to
modify the activity of FLT3 in AML. This is because of
its activity against receptor tyrosine kinase Axl [26].
Furthermore, there are many obstacles researchers
have to endure when trying to find the most efficient
and beneficial FLT3 inhibitor to treat diseases such as
AML. An obstacle includes how FLT3 inhibitors have
shown to select against the activity of receptor c-kit.
C-kit is important in the maintenance and process of
hematopoiesis. When c-kit is not working properly, this
can lead to marrow suppression. By using bone marrow
from healthy donors, the comparison of gilteritnib
and quizartnib was completed to show the difference
in effects of c-kit. It was show that gilteritnib has an
EC50 against wild-type c-kit of 102nM which is two
orders of magnitude greater than that of mutant FLT3.
It did not have much effect on normal hematopoiesis.
These results are shown in Figure 9. This study shows
the potential in gilteritnib and how compared to the
other Flt3 inhibitors, it may be the most useful to use to
further investigation [26].
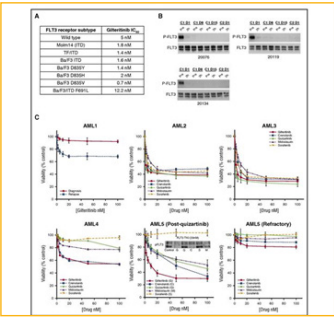
Figure 8: Anti-leukemic activity of gilteritnib [26].
However, there is a study known as the Admiral trial that discusses its results of its phase 3. They released results of patients with acute myeloid leukemia who received gilteritnib therapy in the phase 3 admiral trial [27].As discussed before, gilteritnib acts as an inhibitor of FLT3 and is known for its potency and selectivity. This drug became the first FLT3 inhibitor approved as a single-agent therapy [28]. The results of the admiral showed emerging mutation at relapse in the patients that had FLT3-mutation. It was shown that the gliteritnib stimulated overall survival and resulted in higher remission rates compared to patients with chemotherapy. However, the patients given gliteritnib were shown to develop resistance after their initial response. In this trial, 247 patients were given gilteritnib and 75 of them relapsed during the study. In patients in this study that had FLT3mut+ R/R AML who relapsed on this treatment, Ras/MAPK pathway gene mutations and FLT3 F691L gatekeeper mutations showed most frequently. The presence of a RAS/MAPK pathway gene mutation did not benefit from this drug treatment which could possibly be because of fewer RAS/MAPK pathway gene mutations per patient at baseline than at relapse. The frequency of emergent FLT3 F691 gatekeeper mutations at relapse in patients who received 120-mg/ day gilteritinib in the admiral study was similar to that observed in relapsed patients who received 20- to 450- mg/day gilteritinib [27]. In conclusion, the patients with R/R FLT3mut+ AML, gilteritnib resulted in overall longer survival and higher response rates compared to those with chemotherapy. In addition, the treatment had a favorable safety profile. These results are shown in Figure 10 showing the overall survival in patients treated with the flt3 inhibitor versus chemotherapy [28]. Based on the results of this trial, the FDA approved gilteritnib (Xospata) in November of 2018 for adults with FLT3- posistive acute myeloid leukemia (AML) in the relapsed or refractory setting [29].
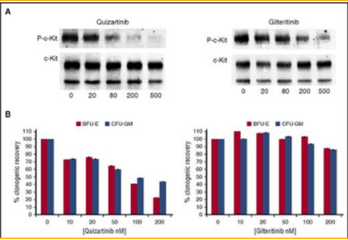
Figure 9: The comparison of quizartinib and gliteritnib, two flt3 inhibitors. This figure shows the comparison of both of their effects on the inhibition of c-Kit by comparing donated bone marrow from healthy patients [26].
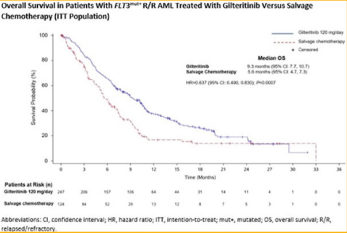
Figure 10: This figure represents patients with FLT3mut+ R/R AML treated with gilteritnib, a flt3 inhibitor, compared to patients treated with chemotherapy [30].
FLT3 inhibitors became to be a great potential for the
treatment of patients with AML since 30% of these
patients had a mutation in FLT3. This first FLT3 inhibitors
were the tyrosine kinase inhibitors as discussed above.
However, TKIs were originally developed for treatment
of solid tumors. These drugs were then surprisingly
found to have activity against FLT3. With further
investigation of these drugs, there have been some that
are designed for the specific purpose of treating patients
with FLT3/ITD AML [31].
More recently, a study states that FLT3-mutated
AML is changing after the approval of midostaurin
for the frontline therapy and gilteritnib for relapsed
or refractory patients. TKI drugs such as gilteritnib,
quizartnib, and sorafenib, which are discussed above,
have shown as positive randomized trials. This predicts
further investigation of the use of FLT3 inhibitors.
These are an important part of therapy for a large subset
of high-risk patients such as those with AML [32].
In another study, the researchers believe that single
agent TKI therapy is not curative and long-term survival
remains low. They implement the idea of combination
therapy. This would include agent that would induce
apoptotic effects with would hopefully enhance cytotoxicity against FLT3mut+ and wild type closes
and potentially delay or prevent drug resistance. They
combined venetoclax with a Flt3 tyrosine kinase
inhibitor, gilteritnib as discussed before. This trial is in
phase 1b clinical trial which means the safety and efficacy
of the combination therapy for patients with relapsed
or refractory AML is completed. The results showed
that of the 15 patients treated in this clinical study, five
of them had WT FLT3 and ten had mutant FLT3. The
conclusions of the study were that combination therapy
of venetoclax and gilteritnib showed to be well tolerated
and showed 90% of patients had blast clearance. The
trial showed that this therapy may be a highly effective
treatment option for patients both with AML FLT3
mutation and those that have relapsed [30].
The effects of using FL, a immunomodulatory cytokine
that acts through flt3, has shown important clinical
use [2]. FLT3 inhibition is continuously evolving and
leading to successes in the treatment of cancers. It is
thought that the combination therapy of chemotherapy
in addition of TKI would be a logical approach in the
treatment of FLT3 mutated AML [31].
Midostaurin is a first-generation FLT3 inhibitor, which are relatively nonspecific for FLT3 as stated above [23]. Researchers explained their phase 1B study of midostaurin with chemotherapy. This study was done in newly diagnosed adult patients with acute myeloid leukemia. They administered midostaurin to twentynine patients on three different dose schedules. For the first two dose schedules that includes receiving 100 mg midostaurin twice a day, 23 of the 29 patients failed to complete their planned therapy causing the discontinuation rate to be high. Adverse events occurred including nausea, vomiting, and diarrhea. Complete remission was achieved by 13 of the 29 patients. The third dose schedule includes patients receiving 50 mg of midostaurin twice a day with chemotherapy or after chemotherapy. The discontinuation rate was lower than the first. This combination therapy showed the midostraurin to be well tolerated in combination with chemotherapy. This study showed the potential of midostaurin with combination of chemotherapy shows improvements in the outcomes and overall survival of patients with FLT3-mutant AML. Although, this study did not account for the influence of stem cell transplantation or gene mutations other that FLT3. But this study did suggest the promising safety of the administration of these drugs and combination therapy for treatment for patients younger than 60 years [33]. Next, they conducted a phase 3 trial to confirm that the addition of midostaurin to chemotherapy would prolong overall survival in a population of 3277 patients from 18-59 years of age with newly diagnosed AML for FLT3 mutations. These patients were randomly selected to receive chemotherapy in addition to either midostaurin or placebo. The results showed that overall survival was significantly longer in the group that received the flt3 inhibitor rather than the placebo [34].
Quizartinib is another FLT3 inhibitor that is active
against both ITD mutant and wild type FLT3. In a phase
1 study of patients with AML it showed favorable results.
Next, the Phase 2 study was completed in order to access
the efficacy and safety of the drug administration. This
include 333 patients in two cohorts. The first cohort
was patients above the age of 60 with AML relapsed in
less than a year. It was shown that 44% of these patients
showed complete remission with quizartinib. However,
some patients experienced fatigue, nausea, anemia,
vomiting etc. The overall experience of the study showed
that quizartinib monotherapy in patients show a high
degree of activity. Many of the patients responded to
the therapy and 8% were able to bridge to potentially
curative hematopoietic stem cell transplantation [35].
Next, quizartinib was compared to the treatment of
chemotherapy in relapsed or refractory FLT3-ITD
AML patients. This was a randomized, controlled,
phase 3 trial. It was shown that the treatment with the
Flt3 inhibitor, quizartnib proved to have a survival
benefit in comparison to chemotherapy. This study as
well highlighted the potential of potent, selective Flt3
inhibitors and becoming a standard medical care for
patients with the poor prognosis of AML [36].
Sorafenib is a multikinase inhibitor that works to inhibit
tumor growth as well as angiogenesis37. It does this by inhibiting intracellular Raf kinases and cell surface
kinase receptors. This drug has been evaluated in
preclinical and clinical trials and has been approved by
the U.S. Food and Drug Administration [38].
Researches discussed and examined the effects of
Sorafenib, another FLT3 inhibitor, and its effects in older
patients with untreated FLT3-ITD mutated AML. They
did this by combining the sorafenib with 5-azacytidine.
This treatment was for those that could not undergo
chemotherapy because of age or fitness. The study
comprised of twenty-seven patients with untreated
AML and all of them had FTL3-ITD mutations. The
study showed that the median survival of patients that
received the combination therapy was 8.3 months of
the entire group. There were three patients that were
responding well so they went on to receive allogeneic
stem cell transplantation. Those who responded had a
higher median overall survival of 9.2 months (Figure
11). It was shown that twelve of the twenty-seven
patients had secondary AML. Furthermore, the study
showed that in older patients with FLT3-ITD mutated
AML have worse results than do wild type FLT3
patients. However, patients with flt3-itd have slowing
been improving in progress of outcomes. Sorafenib was
discontinued because of its toxicity but when combined
with AZA, it was well tolerated. This study had a small
sample-size but their findings showed AZA in addition
to sorafenib shows a great potential in treatment for
elderly patients with FLT3-ITD AML. A larger sample
size would be recommended [39].
Another study examined the effects of the combination
therapy using Sorafenib, Cytarabine, and Idarubicin for
initial therapy in younger patients with AML. First, they
conducted a Phase I study to determine the efficacy
and toxicity of the combination of these drugs. They
treated patients with relapsed AML and chemotherapy
to establish the feasibility of the combination. It was
shown that sorafenib could be safety combined with
chemotherapy. The results showed that the combination
produced a higher complete remission rate as well as
inhibition of FLT3 signaling [40]. This was the final
report of a phase II study. This combination therapy
was able to increase high complete response in patients
with the FLT3 mutation and a one-year probability of
survival of 74%. There were 62 patients involved in this
study and 17 of them had the mutation of FLT3.
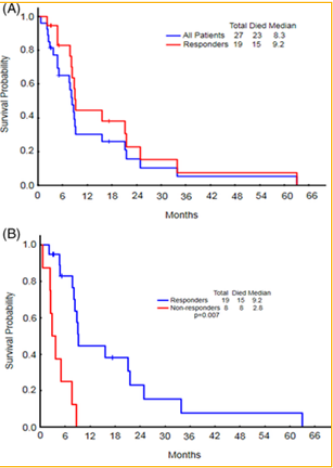
Figure 11: In the study, there were 27 patients that received combination therapy of sorafenib with 5-azacytidine (AZA). This figure shows the comparison of overall survival between all of the patients and the patients who responded. The median overall survival for all 27 patients was 8.3 months whereas the patients that were responders had a longer overall survival of 9.2 months [39].
Unlike other studies, this study showed significantly
shorter overall survival for patients with FLT3 mutation.
This could be due to the increased possibility of patients
able to undergo allogeneic stem cell transplant as a result
of sorafenib addition. The result of this study should be
to better discern the potential role of sorafenib in the
treatment of patients with FLT3-ITD AML [37].
In 2013, a Phase III study was directed on the
administration of sorafenib to newly diagnosed AML
elderly patients 60 years of age or older. They randomly assigned patients to a placebo or sorafenib between
chemotherapy cycles. They found no statistically
significant improvement in event-free survival or overall
survival in the arm treated with sorafenib. Essentially,
they found more adverse effects that occurred in the
treated arm. This study showed the lack of effects of
sorafenib in elderly patients with AML, suggesting it
was not beneficial [41].
A randomized, double-blind, placebo-controlled study
was conducted to access the effects of sorafenib because
of its potential to be an effective drug for the treatment
of AML. Here, they conducted a Phase II trial with
patients of the age 18-60 years old. This study found that
the addition of sorafenib to chemotherapy increased
toxicity but did show to have antileukemic efficacy.
They advise to find a strategy to reduce the toxicity to
determine a future role of this inhibitor in the treatment
of this disease [42].
Another first generation FLT3 inhibitor used is named Sunitinib. They explain how sunitinib was the first FLT3 inhibitor to be used in a clinical setting. It has showed to have equal efficacy against FLT3-itd mutation so it can be used in almost all patients with FLT3 mutation in contrast to quizartinib or sorafenib. This was used in a phase I/II trial is Here, they treated newly diagnosed AML elderly patients over the age of 60 with the presence of FLT3-ITD or tyrosine kinase domain. They found sunitinib added to chemotherapy showed respectable toleration and the complete remission rates and longterm outcome proved to be favorable in comparison to published studies. This showed the need for further investigation on this first generation FLT3 inhibitor in combination with chemotherapy [43].
Crenolanib is a type I FLT3 tyrosine kinase inhibitor. This is a second-generation FLT3 inhibitor. This type I oral inhibitor inhibitors both FLT3-itd and FLT3-tkd mutations. However, this drug does not inhibit c-kit which allows for a full count recovery even when it is combined with chemotherapy. This study reports a phase II trial for the efficacy and the ability to tolerate the drug when it is combined with chemotherapy. They administered the drug in combination with cytarabine and anthracycline induction chemotherapy to patients with newly diagnosed AML. Their results displayed crenolanib to be safely combined at full doses with cytarabine/daunorubicin or cytarabine/idarubicin induction and chemotherapy. This study showed a 88% complete remission rate with full count recovery after one cycle of induction. This allowed allogenic stem cell therapy to be able to be administered on schedule. This trial continues but this phase showed encouraging results of anti-leukemic activity with no relapses of patient younger than 60 with flt3-mutant AML [44].
When creating and exploring new treatment options,
undesirable and unexpected results have the possibility
of occurring. The unexpected results can be harmful
and do the opposite of treating the patient. These need
to be discussed and reviewed in order to prevent them
from occurring again. For example, one study explains
potential consequences of FLT3/ITD AML treatment.
FLT3/AML is a disease that is shown to change
between diagnosis and relapse. Leukemia cells become
more drawn to FLT3 signaling after recurrence after
chemotherapy. It has been shown that after the treatment
of chemotherapy, this leads to a high level of FL in the
plasma during recovery. Since FL acts through FLT3,
when FLT3 in mutated, those high levels of FL from
chemotherapy will maximize its activity in promoting
the survival of leukemia blasts. This could be a large
issue in the treatment of AML since 30% of them are
shown to have the FLT3/ITD mutation. This leads to the
questioning of chemotherapy promoting the relapse of
FLT3/ITD AML. These findings can be used to predict
clinical responses and help design treatments [45].
It is important to discuss potential consequences
to FLT3 inhibitor therapy. These include cutaneous
reactions in patients with AML which include leukemia
cutis, sweet syndrome, infections, and treatment related
effects. One study reports three cases of FLT3 inhibitor
associated neutrophilic dermatoses. These patients
had flt3mut+ AML and participated in clinical trials
for treatment. First, there were seventy-seven patients
in this clinical trial and only three of them developed
confirmed cases of neutrophilic dermatosis. The first
patient was in her 40s and relapsed with FLT3-ITD. She
began to develop tender, red subcutaneous nodules on her back and lower extremities. The second
patient was a man in his seventies that developed
postulonodules on his cheeks, ears, and scalp (Figure
12). Lastly, patient was a woman in her sixties that also
developed tender, red eruptions on her neck and arms.
All of these patients had to stop their treatments of
the FLT3 inhibitors. These patients have had terminal
differentiation of leukemia cells to neutrophils during
their treatment resulting in neutrophilic dermatoses.
This study discussed the importance of dermatologist
awareness during the treatments with FLT3 inhibitors.
This shows a complication of molecular-targeted
therapy of AML and something to be aware of when
proceeding with further investigation [46].
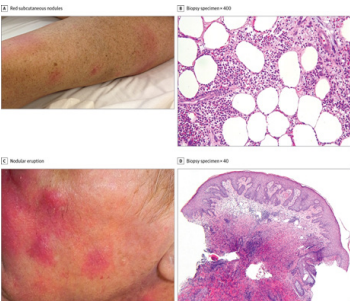
Figure 12: On the left shows the clinical findings in FLT3 inhibitor-associated neutrophilic dermatosis. This includes red subcutaneous nodules on the lower extremities of the patient and nodular eruption of the cheek. On the right-hand side, shows pathologic findings of the same thing confirming the diagnosis of neutrophilic dermatoses [46].
Heat shock proteins (HSPs) are chaperone proteins that help proteins in the regulation of folding/unfolding. These proteins are expressed at low levels in normal physiological conditions but respond to stressful events at high levels. These proteins have shown to be a potential therapeutic target in AML. In a study, they took AML cells from 75 patients. The patients that had FLT3-ITD had an overrepresentation of HSP levels which showed that there was a strong dependence of HSPs in stabilizing FLT3-ITD encoded oncoproteins. This study showed that HSP90 inhibition mediates anti-leukemic effects through direct and indirect activity. They observed an increased frequency of FLT3 mutations in patients with high HSP levels. They believe from their observations that patients with FLT3 mutations have distinct HSP expression profile that supports leukemogenic effect of mutated FLT3. In conclusion, this study showed HSP90 inhibitors had a pro-apoptotic effect for most AML patients with FLT3-ITD [47].
In summary, the effect of FLT3 stimulators and
their role in stem cell maintenance and therapy was
described above. FLT3 is a receptor tyrosine kinase
that is expressed by immature hematopoietic cells. This
receptor is vital in the process of normal hematopoiesis
as well as the immune system. However, patients with
acute myeloid leukemia show to have a mutation in
this receptor. The role of FLT3 in hematopoiesis has
provided information of how FLT3-related leukemias,
like acute myeloid leukemia, can be treated.
Once this receptor, FLT3, is mutated, it can dimerize,
phosphorylate, and activate its kinase domains without
the need for the ligand to bind. This is known as
auto-phosphorylation. This results in the constitutive
activation of FLT3. When this happens, downstream
gene expression is disrupted and can be responsible for
the development of leukemia. This is because normal
FLT3 signaling has shown to increase the survival
and growth of cells. However, the mutation of FLT3
signaling has shown the disruption in differentiation
which results in the cell to transform into a leukemic
blast, therefore, resulting in patients with leukemia.
Patients with the FLT3 mutation have AML which has
a very poor prognosis. Because of this, studies have
brought about the idea of FLT3 inhibitors for a potential
treatment of this disease for those who have that
mutation. There have been positive randomized trials
of the FLT3 inhibitor drugs that highlight the potential
of this treatment in those patients. Many reports have
been identified that shed light on the progess in this
field. The only treatment for AML is chemotherapy which includes many complications include relapse and
toxicity. FLT3 inhibitors now emerge as an important
part of therapy for high risk patients with AML that
have no option of treatment.
The large amount of information on this topic discussed
above has increased knowledge regarding the role
and importance of FLT3 in the process of normal
hematopoiesis. However, since most of the inhibitor
drugs are still in the beginning of their research, more
information and investigation is needed. In addition,
the clinical significance of FLT3 signaling requires
further investigation in its role in leukemia.
1.Grafone T, Palmisano M, Nicci C, Storti S (2012) An
overview on the role of FLT3-tyrosine kinase receptor
in acute myeloid leukemia: Biology and treatment,
Oncology reviews 17:6 https://www.ncbi.nlm.nih.gov/
pubmed/25992210.
2.Wodnar-Filipowicz A (2003) Flt3 ligand: Role in
control of hematopoietic and immune functions of
the bone marrow, Physiology 18: 247-251. https://
www.physiology.org/doi/full/10.1152/nips.01452.2003.
Accessed Nov 19, 2019. doi: 10.1152/nips.01452.2003.
3.Tsapogas P, Mooney CJ, Brown G, Rolink A (2017)
The cytokine Flt3-ligand in normal and malignant
hematopoiesis, Int J Mol Sci 18: https://www.ncbi.nlm.
nih.gov/pmc/articles/PMC5485939/.
4.Gilliland DG, Griffin JD (2002) The roles of
FLT3 in hematopoiesis and leukemia, Blood 100:
1532-1542 https://ashpublications.org/blood/
article/100/5/1532/106333/The-roles-of-FLT3-inhematopoiesis-and-leukemia.
5.Mooney CJ, Cunningham A, Tsapogas P, Toellner
KM, Brown G (2017) Selective expression of Flt3 within
the mouse hematopoietic stem cell compartment, Int J
Mol Sci 18: 1037.
6. Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi
S (2009) FLT3-ITD up-regulates MCL-1 to promote
survival of stem cells in acute myeloid leukemia via
FLT3-ITD-specific STAT5 activation, Blood 114: 5034-
5043 https://doi.org/10.1182/blood-2008-12-196055.
7. Small D (2008) Targeting FLT3 for the treatment
of leukemia, Seminars in Hematology 45:S17-S21
http://www.sciencedirect.com/science/article/pii/
S0037196308001236.
8. Li GX, Wang L, Yaghmour B, Yaghmour G,
Ramsingh G (2018) The role of FLT3 inhibitors as
maintenance therapy following hematopoietic stem
cell transplant, Leukemia Research Reports 10:26-36.
https://www.sciencedirect.com/science/article/pii/
S2213048918300499.
9. Stem cell transplant: Allogenic prodedure,options &
recovery (2018) Cancer Treatment Centers of America
Web site, https://www.cancercenter.com/treatmentoptions/hematologic-oncology/allogenic-stem-celltransplant.
10. Kottaridis PD, Gale RE, Linch DC (2003)
Flt3 mutations and leukaemia, British Journal of
Haematology 122: 523-538 https://onlinelibrary.wiley.
com/doi/abs/10.1046/j.1365-2141.2003.04500.x.
11. Levis MJ, Chen Y, Hamadani M, Horowitz MM, Jones
RJ (2019) FLT3 inhibitor maintenance after allogeneic
transplantation: Is a placebo-controlled, randomized
trial ethical? J Clin Oncol 37: 1604-1607 https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC6804888/.
12. Gilliland DGP, Griffin JD† (2002) Role of FLT3 in
leukemia, 9: 274-281.
13. Stam RW, den Boer ML, Schneider P (2005) Targeting
FLT3 in primary MLL-gene-rearranged infant acute
lymphoblastic leukemia, Blood 106: 2484-2490. https://
ashpublications.org/blood/article/106/7/2484/21682/
Targeting-FLT3-in-primary-MLL-gene-rearranged.
14. McKenna HJ, de Vries P, Brasel K, Lyman SD,
Williams DE (1995) Effect of flt3 ligand on the ex vivo
expansion of human CD34+ hematopoietic progenitor
cells, Blood 86:3413-3420. https://www.ncbi.nlm.nih.
gov/pubmed/7579445.
15. Sitnicka E, Bryder D, Theilgaard-Mönch K,
Buza-Vidas N, Adolfsson J, Jacobsen SEW (2002)
Key role of flt3 ligand in regulation of the common
lymphoid progenitor but not in maintenance of the
hematopoietic stem cell pool, Immunity 17: 463-472
http://www.sciencedirect.com/science/article/pii/
S1074761302004193.
16. Whartenby KA, Small D, Calabresi PA (2008) FLT3
inhibitors for the treatment of autoimmune disease,
Expert Opin Investig Drugs 17: 1685-1692 https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC4882767/.
17. Tussiwand R, Onai N, Mazzucchelli L, Manz MG
(2005) Inhibition of natural type I IFN-producing and
dendritic cell development by a small molecule receptor
tyrosine kinase inhibitor with Flt3 affinity, J Immunol
175: 3674-3680.
18. Whartenby KA, Calabresi PA, McCadden E (2005)
Inhibition of FLT3 signaling targets DCs to ameliorate
autoimmune disease, Proc Natl Acad Sci U S A 102:
16741-16746.
19. Teshima T, Reddy P, Lowler KP (2002) Flt3 ligand
therapy for recipients of allogeneic bone marrow
transplants expands host CD8 alpha (+) dendritic
cells and reduces experimental acute graft-versus-host
disease, Blood 99: 1825-1832 https://www.ncbi.nlm.
nih.gov/pubmed/11861301.
20. Larrosa-Garcia M, Baer MR (2017) FLT3 inhibitors
in acute myeloid leukemia: Current status and future
directions, Mol Cancer Ther 16: 991-1001 https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC5600895/.
21. Garcia JS, Stone RM (2017) The development of
FLT3 inhibitors in acute myeloid leukemia, Hematol
Oncol Clin North Am 31: 663-680.
22. Hassanein M, Almahayni MH, Ahmed SO, Gaballa
S, El Fakih R (2016) FLT3 inhibitors for treating acute
myeloid leukemia, Clin Lymphoma Myeloma Leuk 16:
543-549.
23. Sutamtewagul G, Vigil CE (2018) Clinical use of
FLT3 inhibitors in acute myeloid leukemia, OncoTargets
and therapy 11: 7041-7052 https://www.ncbi.nlm.nih.
gov/pubmed/30410361.
24. Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T,
Levis M (2010) FLT3-mutant allelic burden and clinical
status are predictive of response to FLT3 inhibitors in
AML, Blood 115: 1425-1432 https://www.ncbi.nlm.nih.
gov/pubmed/20007803.
25. Knapper S, Russell N, Gilkes A (2017) A randomized
assessment of adding the kinase inhibitor lestaurtinib to
first-line chemotherapy for FLT3-mutated AML, Blood
129:1143-1154 https://www.ncbi.nlm.nih.gov/pmc/
articles/PMC5364440/.
26. Lee LY, Hernandez D, Rajkhowa T (2017) Preclinical
studies of gilteritinib, a next-generation FLT3 inhibitor,
Blood 129:257-260. https://www.ncbi.nlm.nih.gov/
pmc/articles/PMC5234222/.
27. Smith CC, Levis MJ, Perl AE ( 2019) Emerging
mutations at relapse in patients with FLT3-mutated
relapsed/refractory acute myeloid leukemia who
received gilteritinib therapy in the phase 3 admiral trial,
Blood 134:14.
28. Perl A1, Martinelli G2, Cortes J3 (2019) Gilteritinib
significantly prolongs overall survival in patients with
flt3-mutated (flt3mut+) relapsed/refractory (r/r) acute
myeloid leukemia (aml): Results from the phase 3
admiral trial, 3: 392-393.
29. Single-agent FLT3 inhibitor gets FDA nod for acute
leukemia, Updated 2018 https://www.medpagetoday.
com/hematologyoncology/leukemia/76574.
30. Perl AE, Daver NG, Pratz KW (2019) Venetoclax in
combination with gilteritinib in patients with relapsed/
refractory acute myeloid leukemia: A phase 1b study,
Blood 134: 3910-3910 https://www.bloodjournal.
org/blood/article/134/Supplement_1/3910/424199/
Venetoclax-in-Combination-with-Gilteritinib-in.
31. Grunwald MR, Levis MJ (2013) FLT3 inhibitors for
acute myeloid leukemia: A review of their efficacy and
mechanisms of resistance, Int J Hematol 97: 683-694.
32.Perl AE (2019) Availability of FLT3 inhibitors: How
do we use them? Blood 134: 741-745.
33. Stone RM, Fischer T, Paquette R (2012) Phase IB
study of the FLT3 kinase inhibitor midostaurin with
chemotherapy in younger newly diagnosed adult
patients with acute myeloid leukemia, Leukemia 26:
2061-2068 https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC4118284/.
34. Stone RM, Mandrekar SJ, Sanford BL (2017)
Midostaurin plus chemotherapy for acute myeloid
leukemia with a FLT3 mutation, N Engl J Med 377:
454-464 https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5754190/.
35. Cortes J, Perl AE, Döhner H (2018) Quizartinib, an
FLT3 inhibitor, as monotherapy in patients with relapsed
or refractory acute myeloid leukaemia: An open-label,
multicentre, single-arm, phase 2 trial, The Lancet
Oncology 19: 889-903 https://www.sciencedirect.com/
science/article/pii/S1470204518302407.
36.Cortes JE, Khaled S, Martinelli G (2019) Quizartinib
versus salvage chemotherapy in relapsed or refractory
FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A
multicentre, randomised, controlled, open-label, phase
3 trial, The Lancet Oncology 20: 984-997.
37. Ravandi F, Yi CA, Cortes JE (2014) Final report of
phase II study of sorafenib, cytarabine, and idarubicin
for initial therapy in younger patients with acute
myeloid leukemia, Leukemia 28: 1543-1545 https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC4091714/.
38. Ravandi F, Yi CA, Cortes JE (2014) Final report of
phase II study of sorafenib, cytarabine, and idarubicin
for initial therapy in younger patients with acute
myeloid leukemia, Leukemia 28: 1543-1545 https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC4091714/.
39. Ohanian M, Garcia-Manero G, Levis M (2018)
Sorafenib combined with 5-azacytidine in older
patients with untreated FLT3-ITD mutated acute
myeloid leukemia, Am J Hematol 93:1136-1141 https://
doi.org/10.1002/ajh.25198.
40. Farhad Ravandi, Jorge E. Cortes, Daniel Jones (2010)
Phase I/II study of combination therapy with sorafenib,
idarubicin, and cytarabine in younger patients with acute
myeloid leukemia, Journal of Clinical Oncology 28:
1856-1862 http://jco.ascopubs.org/content/28/11/1856.
abstract.
41. Serve H, Krug U, Wagner R (2013) Sorafenib in
combination with intensive chemotherapy in elderly
patients with acute myeloid leukemia: Results from a
randomized, placebo-controlled trial, J Clin Oncol 31:
3110-3118.
42. Röllig C, Serve H, Hüttmann A (2015) Addition of
sorafenib versus placebo to standard therapy in patients
aged 60 years or younger with newly diagnosed acute
myeloid leukaemia (SORAML): A multicentre, phase
2, randomised controlled trial, Lancet Oncol 16: 1691-
1699.
43. Fiedler W, Kayser S, Kebenko M (2015) A phase I/II
study of sunitinib and intensive chemotherapy in patients
over 60 years of age with acute myeloid leukaemia and
activating FLT3 mutations, Br J Haematol 169: 694-700.
44. Wang ES, Stone RM, Tallman MS, Walter RB,
Eckardt JR, Collins R (2016) Crenolanib, a type I FLT3
TKI, can be safely combined with cytarabine and
anthracycline induction chemotherapy and results in
high response rates in patients with newly diagnosed
FLT3 mutant acute myeloid leukemia (AML), Blood
128: 1071-1071 https://ashpublications.org/blood/
article/128/22/1071/96226/Crenolanib-a-Type-I-FLT3-TKI-Can-be-Safely.
45. Levis M (2011) FLT3/ITD AML and the law of
unintended consequences, Blood 117: 6987-6990.
46. Varadarajan N, Boni A, Elder DE (2016) FLT3
Inhibitor-Associated neutrophilic dermatoses, JAMA
Dermatology 152: 480-482 http://dx.doi.org/10.1001/
jamadermatol.2015.6121.
47. Reikvam H, Hatfield KJ, Ersvær E (2012) Expression
profile of heat shock proteins in acute myeloid
leukaemia patients reveals a distinct signature strongly
associated with FLT3 mutation status - consequences
and potentials for pharmacological intervention,
Br J Haematol 156: 468-480 https://doi.org/10.1111
/j.1365-2141.2011.08960.
