Author(s): Sheharyar Minhas*, Ahmed Minhas, Maira Malik, Sana Javaid and Sunil Dhar
Digoxin is an antiarrhythmic medication that has been used to manage chronic heart failure. Despite more than 200 years of research, the role of digoxin in patients with heart failure and sinus rhythm remains controversial. Recent studies have shown digoxin to reduce heart failure associated morbidity without having any effect on mortality. The purpose of our study was to study the impact of digoxin on mortality in heart failure patients in sinus rhythm.
Heart failure (HF) is a chronic cardiovascular condition that results from common cardiovascular conditions and age-related changes in cardiovascular function and structure. The incidence and prevalence of HF increases with age for both sexes. Approximately 915,000 new cases of HF are diagnosed each year in the United States [1]. HF has now become the most common reason for hospitalizations in elderly [2]. Due to the aging population the total healthcare cost of patients with heart failure exceeds $30 billion annually and is expected to increase to more than $70 billion by the year 2030 [3]. Myocardial infarction (MI) is the leading cause of heart failure and it occurs as a result of inability of the heart to respond to physiologic (i.e. exercise) or pathologic (i.e. sepsis, myocardial ischemia) stress. In HF the heart cannot pump enough blood to adequately oxygenate the tissues [4]. Currently about 6.5 million adults in the United States have HF [5]. In United States 1 in 8 deaths in 2017 was contributed to the HF [6]. Lifestyle factors that contribute to the HF include unhealthy diet high in saturated and trans-fat, sedentary lifestyle, and tobacco use. Comorbidities associated with heart failure include coronary artery disease (CAD), hypertension, diabetes mellitus, hyperlipidemia, and obesity [7].
HF treatment commonly includes the use of angiotensinconverting-enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), beta-blockers, and diuretics. Digoxin is an antiarrhythmic medication that has been used to manage chronic heart failure (CHF). Digoxin increases cardiac contractility and enhances parasympathetic tone by depressing activity at sinoatrial and atrioventricular nodes. Despite more than 200 years of research, the role of digoxin in patients with CHF and sinus rhythm remains controversial. Recent studies have shown digoxin to reduce CHF associated morbidity without having any significant effect on mortality. Some trials have indicated that digoxin improves the quality of life by improving exercise tolerance, reducing symptoms, and preventing hospitalizations [8].
The Digitalis Investigation Group (DIG) trial was a randomized, double-blinded, multicenter trial conducted from February 1991 to August 1993 [9]. The purpose of the main DIG trial was to study the effect of digoxin on hospitalization and on all-cause mortality in heart failure patients with normal sinus rhythm and left ventricular ejection fraction (LVEF) ≤0.45. Patients with LVEF ≤0.45 were randomly assigned to digoxin (n=2297) or placebo (n=3403) group in addition to ACEIs and diuretics. Median dose of digoxin 250mcg was given daily and subjects were followed for 37 months on average. Mortality was unaffected in the main trial as there were 1181 deaths (34.8%) in the digoxin group and 1194 deaths (35.1%) in the placebo group (p=0.80). There were fewer overall hospitalizations in the digoxin group than in the placebo group, and fewer patients were admitted to the hospital for worsening HF (26.8% vs 34.7%; p<0.001). Use of digoxin reduced the rate of both overall and worsening heart failure hospitalizations but digoxin use did not reduce overall mortality. Our study used the National Institute of Health (NIH) dataset to reproduce original results of the DIG trial. Our study’s aim was to determine whether digoxin has an effect on the mortality (in months) over a 3-5 year time period. Our study considered New York Heart Association (NYHA) HF severity as well as known risk factors for CHF such as age, race, and previous MI.
Patients were enrolled at multiple clinical centers in the United States and Canada, and NIH database was used for the study. The original study was approved by the institutional review board at each participating center. Patients were eligible for the study if they had HF with LVEF ≤0.45 and were in normal sinus rhythm. The diagnosis of heart failure was based on current or past clinical symptoms such as exercise limitation, dyspnea, or orthopnea signs such as elevated jugular venous distention, rales or lower extremity edema; or radiological evidence of pulmonary congestion. The LVEF was assessed by transthoracic echocardiogram. There were 6800 subjects at base-line but 7 subjects were removed from the study for missing data for any of the covariates. Analysis was performed on the remaining 6793 subjects (Figure 1). HF patients with LVEF ≤0.45 were randomly assigned to digoxin group (n=3392) or placebo group (n=3401). The primary outcome studied in the study was mortality. Adjusted survival curve for the treatment group by NYHA functional class, adjusted for age, sex, race, and previous MI was also studied.
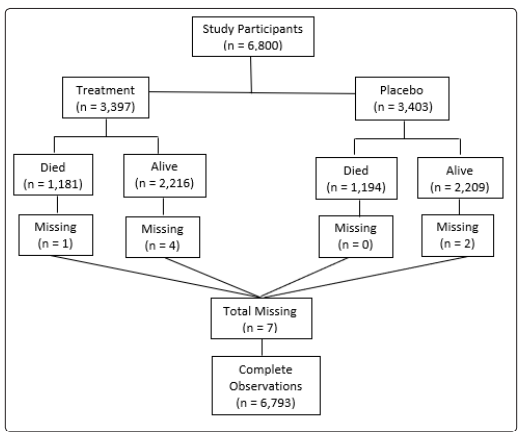
Figure 1: Central illustration: Selection criteria for data analysis
All the analysis was performed with an intention-to-treat basis with two-sided P values. Log-log plots and Goodness of Fit testing with Schoenfeld residuals was used to evaluate the Cox proportional hazards (PH) assumption for each of the covariates including the NYHA class, age, sex, race, and previous MI. In comparing the digoxin and placebo groups, we estimated the hazard ratio associated with an event and calculated the 95% confidence interval from the Cox PH model. The Cox PH model was also used to test interaction between the study assignments and the covariates that included the age, race, sex, and previous MI. No interaction assumption for the stratified Cox models was assessed using the likelihood ratio (LR) test. Kaplan-Meier analysis was used to construct the unadjusted and adjusted survival probability plots.
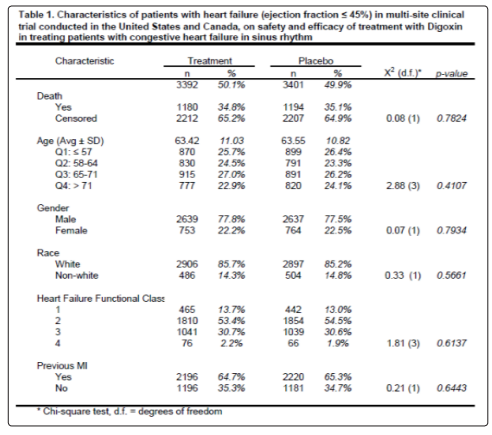
There were no significant differences in base-line characteristics of HF patients with normal sinus rhythm with LVEF ≤0.45 between the 3392 patients assigned to digoxin group and the 3401 patients assigned to placebo group (Table 1). The mean duration of followup time was 38.4 months from randomization to either death or last follow up. The mean duration of follow-up time for the placebo and treatment group was 38.68 and 38.23 months, respectively. The unadjusted median survival time for the placebo group was 58.37 months. The median survival time for the treatment group could was not reported because the group did not reach 50% survival probability during the study.
Unlike the NYHA class, neither the treatment, age, gender, race, or previous MI covariates violated the PH assumption using the Schoenfeld residual p-values (Table 2). The log-log plots for all covariates including the treatment, age, gender, race, NYHA, and previous MI did not result in any major violation of the PH assumption (Figures 2-7). The likelihood ratio (LR) test for no interaction assumption was insignificant (p=0.54) so we assumed there were no interactions present.
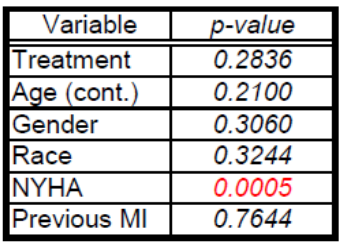
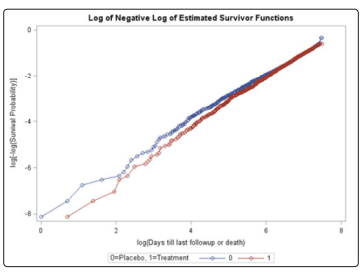
Figure 2: Log-log plot for treatment
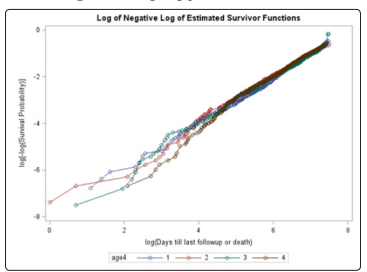
Figure 3: Log-log plot for age quartiles
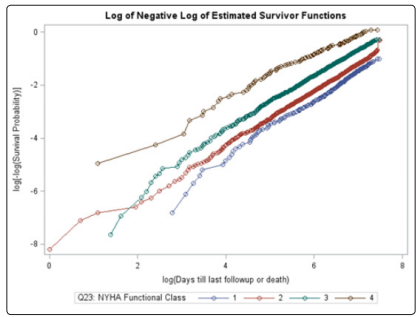
Figure 4: Log-log plot for NYHA functional class
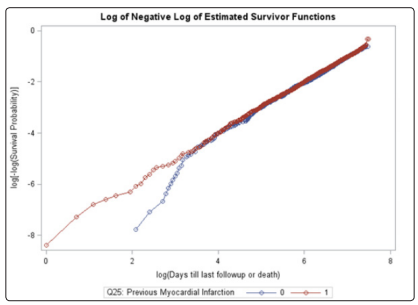
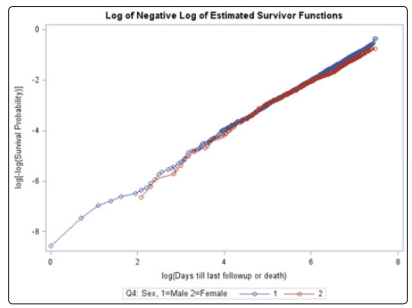
Figure 7: Log-log plot for sex
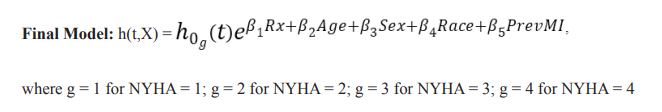
There were 1180 deaths in the digoxin group (34.77%) and 1194 deaths in the placebo group (35.09%). The adjusted hazard ratio (AHR) for the treatment group was 0.981 (p=0.6425) which was not statistically significant (Table 3). The unadjusted Kaplan-Meier plot for treatment groups is shown in (Figure 8). The adjusted survival curves for treatment group by NYHA functional class adjusted for age, sex, race and previous MI is shown in (Figure 9).
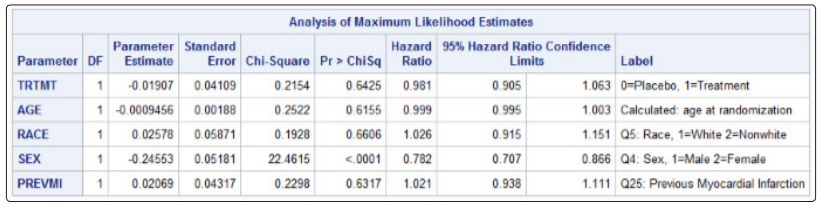
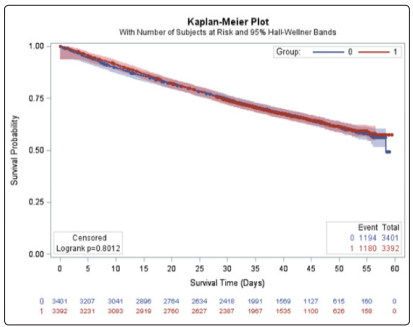
Figure 8: Unadjusted Kaplan-Meier plot for treatment groups
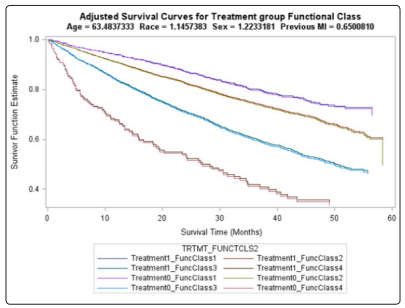
Figure 9: Final adjusted survival curve for treatment group by NYHA functional class
Our research studied the effect of digoxin on time to death in HF patients with LVEF ≤0.45 who were in normal sinus rhythm. The adjusted HR for treatment was not statistically significant although there were fewer deaths in the digoxin group. Therefore, we found that digoxin had no effect on overall mortality in treating patients with HF. After controlling for age, gender, race, previous MI, and NYHA functional class, we found no difference in the hazard of death between those receiving digoxin and those receiving placebo. The final adjusted survival curve clearly demonstrated that treatment had no effect on the survival for all 8 treatment and NYHA functional class combinations, after controlling for the other covariates (Figure 9).
Our study replicated findings of the DIG trial and found no effect of digoxin in reducing the overall mortality. The DIG trial found no mortality benefit of digoxin but found reduction in the hospitalization rate. Previous studies such as the DIG trial and smaller studies, such as the Randomized Assessment of Digoxin on Inhibitors of the Angiotensin-Converting Enzyme (RADIANCE) and Prospective Randomized Study of Ventricular Failure and the Efficacy of Digoxin (PROVED) trials, have suggested that worsening HF and hospitalization occurred less frequently in patients treated with digoxin [10,11]. Our study assessed the effect of digoxin on mortality but we did not study the impact of digoxin on worsening HF symptoms and hospitalizations. Although our study showed no effect of digoxin on mortality, previous studies of inotropic agents, such as beta-agonists, dobutamine, and milrinone, have demonstrated increased mortality [12,13].
There were several limitations of our study. There were potentially minor graphical violations of the PH assumption for all covariates using the log-log plots. However, we used Schoenfeld residuals instead as they are less subjective in interpretation and have commonly been used in the research. There might have been residual unmeasured confounding factors. Our findings can only suggest an association, rather than a causality of no benefit or benefit with digoxin treatment. Further studies may need to confirm our findings and further expand to include the effect of digoxin on the length of stay and hospitalizations. In future, studies might need to explore the effect of digoxin on mortality in different age groups as well as with various comorbidities such as hypertension, diabetes mellitus, and obesity. In conclusion, digoxin had no effect on overall mortality in HF patients with LVEF less than 0.45 who were in normal sinus rhythm.
After controlling for age, gender, race, previous MI, and NYHA functional class, we found no difference in the hazard of death between those receiving digoxin and those receiving placebo. Our study demonstrated that digoxin did not reduce the overall mortality and has no survival benefit in the management of chronic heart failure.
