Author(s): Ayat M Abdaltawab, Nagwa A. Meguid, Eman M. Khaled, Adel Hashish, Mohamed H. Bahary, Mohamed S. Taha*
Oxidative stress is a common feature in nearly all neurodegenerative disorders including autism spectrum disorder (ASD). ASD is behaviorally defined disorder associated with abnormalities in social communication, behavior, language and perception. Chronic exposure to different common stress factors like immune inflammation, malnutrition, alcohol, abuse and drugs induce congenital and behavioral impairment. To the best of our knowledge this study is the first exploring the rate of oxidative stress and its effect on telomere length simultaneously in Egyptian autistic children. In this study we investigated the effect of oxidative stress associated with ASD. Patients were selected based on full clinical and neurological examination and the severity of autism evaluated using childhood autism rating scale (CARS) and Autism Diagnostic Interview–Revised (ADI™-R). Expectedly we found the majority of autism affected children were males, and consanguinity rate between the ASD families was ~ 27% while it was completely absent in the control subjects. We found a highly significant correlation between ASD and low levels of total glutathione (P<0.001) compared to controls. We reported a remarkable highly significant correlation between ASD children and shorter relative telomere length (P<0.001). Moreover we identified a correlation between telomere length and behavioral characteristics in autistic children represented by social interaction impairment (ADI-R 1st domain) (r= - 0.354, P = 0.025). Here we conclude that oxidative stress represented by low glutathione level is an early sign of autism, and is affecting the telomere length that protecting the genetic material. Behavioral abnormalities associated with autism are dependent on telomere length.
Autism spectrum disorder (ASD) is a complex neurodevelopmental syndrome, which usually occurs at early childhood (~2 years of age). In 1943 Autism first identified by Leo Kanner as an innate inability to create normal, biologically determined, emotional contact with others [1,2]. In general, ASD is characterized by pervasive deficits in social interaction, impairments in verbal and nonverbal communication, and stereotyped patterns of interests and activities [3]. ASD varies in severity and the clinical phenotype reflects multifactorial background [4]. ASD prevalence was 18.5 per 1,000 (one in 54) children aged 8 years (ADDM Network sites) in the United States [5]. ASD prevalent by about 4 times higher in boys than in girls [5-7]. The estimated prevalence of ASD among the neurodevelopmentally disabled children in Egypt was to be 33.6% [8]. Recently both telomere length and oxidative stress are considered a promising biomarker for early a neurodevelopmental evaluation [9].
Telomeres are nucleoprotein structures localized at the end of chromosomes that function to protect chromosomes from degradation. Telomeres are formed from repetitive noncoding deoxynucleotide sequences composed of conserved sequence of TTAGGG and associated proteins located at chromosomal ends [10]. This telomeric repeats synthesized by telomerase activity [11]. Telomere length (TL) is affected by both genetic and environmental factors, and shortening of telomeres is associated with multiple neuropsychiatric disorders, early life stress, and age-related cognitive dysfunction [11,12].Considering that telomere shortening has been associated with some psychiatric diseases, there is a possibility that dysfunctional telomeres might be involved in autism pathogenesis and that altered telomere length may be associated with childhood autism [13].
Glutathione (GSH) is a tripeptide, γ-L-glutamyl-L cysteinyl glycine, ubiquitously exist in all mammalian tissue types and crucially in liver tissue with high concentrations [14]. GSH is considered as the most abundant non-protein thiol that defends against oxidative stress. GSH is also a key determinant of redox signaling, vital in detoxification of xenobiotics, modulates cell proliferation, apoptosis, immune function, and fibrogenesis [15]. Glutathione can exist in either thiol-reduced (GSH) or disulfideoxidized (GSSG) forms. The antioxidant reaction of GSH can be summarized in two oxidation and reduction reactions, where the oxidation of GSH to GSSG is accompanied by the reduction of hydrogen peroxide and lipid peroxide. GSSG in turn is reducedback to GSH by GSSG reductase at the expense of NADPH, forming a redox cycle [15]. In brain, glutathione play an important cellular protective function by scavenging reactive oxygen species (ROS) and consequently is involved in various cellular survival pathways in response to oxidative stress [16]. To the best of our knowledge this study is the first to investigate the rate and progression of oxidative stress in Egyptian autistic children, by analyzing the tGSH and RTL.
Subjects: This study included 80 children, 40 of them are affected by childhood ASD and the other 40 are healthy with no history of any major neurodegenerative disease in their families. ASD children were recruited from autism disorders clinic. Autistics diagnosed by a senior clinician in neurology and human genetics. Patients’ consents were read, fully comprehended and signed by tutoring parents of the recruited pediatric subjects. The typicallydeveloping (TD) controls sampled from one common kindergarten with no abnormal histories of motor, language, or social developmental disorders, as determined according to reports of parents. Children in both groups were matched in age (3 - 6 years) and sex. Detailed history of patients has been recorded, including three generation pedigree analysis, pregnancy history, perinatal history, developmental history, similarly affected family members, and age of onset of presenting manifestation. Clinical diagnosis performed based on the criteria for autistic disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-IV) [17,18]. And Autism Diagnostic InterviewRevised (ADI-R) [19]. The Childhood Autism Rating Scale (CARS) was conducted for assessing autism severity [20].The Autism Diagnostic Interview - Revised (ADI-R) was employed on three domains: (Qualitative abnormalities in reciprocal social interaction), (Qualitative abnormalities communications), and (Restricted repetitive & stereotyped pattern of behavior). Patient with other neurodegenerative diseases e.g. fragile-X syndrome, Asperger’s syndrome, Rett syndrome, and patients with childhood schizophrenia have been excluded from this study
Telomere Length Measurements: Blood samples were collected into EDTA tube (BD Vacutainer®) by following standard laboratory procedure. Samples were centrifuged at 1200 rpm for 10 minutes. Cells pellet were used for DNA isolation from leukocyte cell using Qiagen DNeasy Blood & Tissue isolation kit (Qiagen, Germany). Isolated DNA samples were stored at -20C for later relative telomere length analysis (RTL). RTL was measured by the quantitative real-time polymerase chain reaction (PCR) method originally described in details elsewhere [21]. Real time PCR was performed using DTlite Real-Time PCR System (DNA technology, Russia). Using the same primers and protocol previously described in [22]. A comparative quantitation approach was used to measure the telomere length regarding a relative ratio (T/S ratio) of telomere (T) repeat copy number to a single copy of reference gene (S) copy number.
Glutathione Concentration Measurements: Blood samples were collected using heparin as anticoagulant. Total glutathione level was detected in blood erythrocytes of patient and control samples simultaneously by following the manufacturer protocol using OxiSelect TM Total Glutathione (GSSG/GSH) Assay Kit (STA-312) (Cell Biolabs, USA).
Statistical Analysis: Recorded data were analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Comparing proportion was done using Pearson’s correlation coefficient (r) and regression analysis. Receiver operating characteristic (ROC curve) analysis was used to find out the overall sensitivity and specificity. All P-values were two-sided and considered statistically significant at P*<0.05, and highly significant at P**0.001.
The present study included 80 children with age range 2-6 years old. They divided equally into two groups autistics and controls, each group contain 40 children. The autistic group (ASD children) was evaluated according to the criteria defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-IV), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI Tm -R). The control group is healthy children with no history of any associated diseases. Both groups are matched in age and sex, the autistics were (70% males & 30% females) with age mean of 4.28±1.09. While the control group includes (62.5% males & 37.5% females) with age mean of 4.54±0.99. The parents’ consanguinity marriage crucially reported in autistic group.
Autistics were categorized into three categories according to the severity of autism associated symptoms based on the CARS score. About two third of autistics (62.5%) had mild to moderate autism with CARS score of (30 to 37), 20% with sever autism (CARS score >37) and 17.5% with mild autism (CARS score = 30) (Fig 1A). in addition to CARS scores, the autistics were reevaluated clinically by Autism Diagnostic Interview-Revised (AIDR). Both methods showed a similar results for ASD symptoms classification, and we found a significant direct correlation (R2 = 0.376 & P<0.001) as shown in the linear regression curve (Figure 1B). Demographic and clinical characteristics including height, weight and head circumference values were recorded for autistic group. We didn’t find a significant difference in the severity of symptoms between patients based on the demographic and clinical characteristics e.g. sex, height, weight or head circumference.
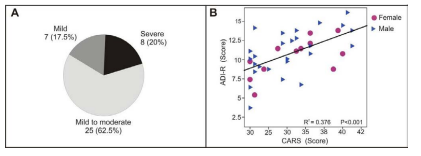
Figure 1: Clinical analysis of autistic group: A) three categories of autism severity based on CARS score, where 7 patients (17.5%) were mild with CARS score (=30), 25 patients (62.5%) were moderate to severe with CARS score of (30-37) and 8 patients (20%) were severe with CARS score of (38-60). B) Scatter plot showing a linear regression and a significant correlation between CARS scores and the mean of the three domains of ADI-R (Qualitative abnormalities in reciprocal social interaction & Qualitative abnormalities communications, and Restricted repetitive with stereotyped pattern of behavior) scores for patient group (R2 =0.376 & P<0.001).
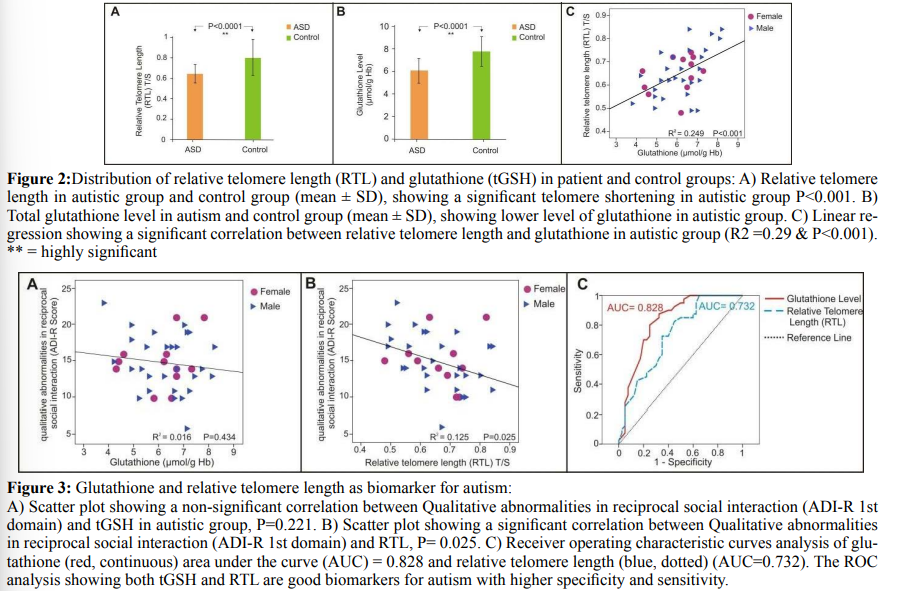
Relative Telomere Length And Glutathione Levels In Autism The mean of relative telomere length (T/S) in the ASD group (mean ± SD) (0.66 ± 0.11) was significantly shorter than the mean RTL in controls (0.8 ± 0.18), (P < 0.001) (Figure 2A & Table 1).
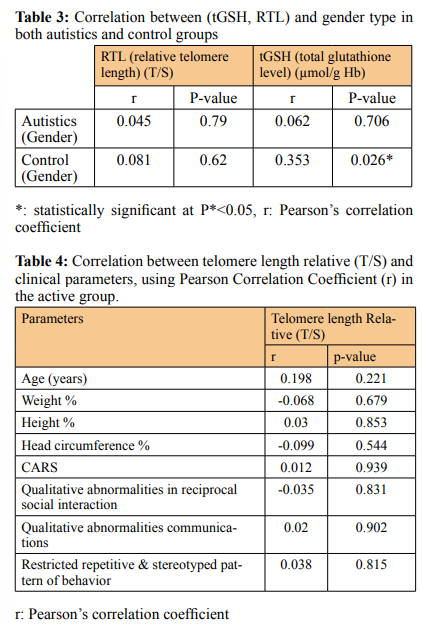
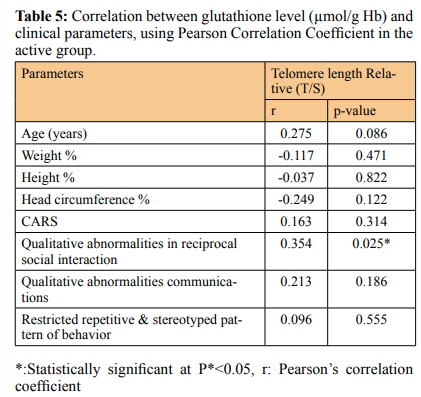
Similarly the ASD group showed a lower tGSH level (6.21 ± 1.12) in comparison to controls (7.76 ± 1.32), (P < 0.001) (Figure 2B & Table 2). The mean of RTL in the autistic females (0.65 ± 0.09) was significantly shorter than those females in control group (0.78 ± 0.16), P= 0.025 and the tGSH level for the females in ASD group was (6.11 ± 1.13) less than those females in control group (7.17 ± 1.23), (P = 0.03). In the same way the males in ASD group showed shorter RTL (0.66 ± 0.11) than the males in control group (0.81 ± 0.19), p<0.001, and the tGSH level was much lower (6.26±1.14) among males in ASD versus those in controls (8.12 ± 1.27), p<0.001 (Table 1&2). There is no correlation between gender identity and RTL in both autistic and control groups (r = 0.045, P = 0.79 & r = 0.08, P = 0.62) respectively. Although the tGSH had a significant correlation with gender in control group only (r= 0.35, P= 0.026) (Table 3). RTL and tGSH showed a direct positive correlation in the ASD group using linear regression analysis and Pearson correlation (r = 0.499, P**=0.001) (Figure 2C). In autistic group the RTL and tGSH showed no correlation with age (r = 0.198, P= 0.221) & (r = 0.275, P*=0.086) respectively. The severity of autism symptoms based on CARS score hadn’t any correlation with RTL or tGSH (Table 1&2). Similarly RTL and tGSH levels were both not correlated to patient height, weight or head circumference (Tables 4&5). RTL showed an inverse correlation with qualitative abnormalities in reciprocal social
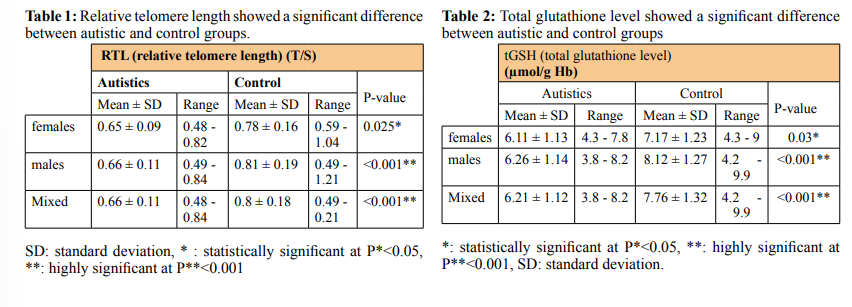
RTL and Glutathione Level as Biological Biomarker in Autism Both the RTL and tGSH were evaluated as biological biomarkers using statistical analysis and receiver operating characteristics (ROC) curves to analyze the sensitivity and specificity of both biomarkers for autism. RTL (AUC = 0.732, (95% CI 0.623 - 0.841), P < 0.001, tGSH (AUC = 0.828 (95% CI 0.735 - 0.92), P < 0.001.
It was reported that both oxidative stress and mitochondrial dysfunction are common features of neurodegeneration [23-25]. In this study, we analyzed the possible association of oxidative stress with one of the most common neurodevelopmental disorders (Autism spectrum disorder, ASD) among Egyptian children. Autistics in this study were evaluated using the Childhood Autism Rating Scale (CARS) for reconfirming the diagnosis of ASD and to assess the severity of ASD symptoms (Figure 1A). In addition to, using the Autism Diagnostic Interview - Revised (ADI-R). ADI-R is a highly recognized evaluative measure for diagnosing Autism Spectrum Disorder (ASD). The ADI-R provides a helpful standardized approach to gathering diagnostic information. In this study, abnormal restricted repetitive & stereotyped patterns of behavior have the highest percentage of cases. In addition, we found that severe CARS is associated with the highest percentage of restricted repetitive & stereotyped patterns of behavior [26]. Consequently, we noted a significant direct correlation between CARS and ADI-R scores in the patient group (Figure 1B).
Here we evaluated the peripheral blood leukocytes relative telomere length as a biomarker for oxidative stress in autism. Telomeres are nucleoprotein structures at the end of chromosomes and are required for integrity and stability of chromosome ends and prevent them from being recognized as DNA double-strand breaks, through their binding with an adequate amount of a sixunit protein complex (shelterin) [27]. Shelterin protein has a specific and crucial role in telomere maintenance and function. Shortening or absence of telomere due to age or oxidative stress leading to a subsequent decrease in the amount of shelterin protein at chromosome ends and further increasing the vulnerability of DNA to degradation and instability [28,29]. Our results are confirming that relative telomere length (RTL) (telomere length in base pair compared to a reference gene (T/S)) is shorter in autistics compared to the control group (Figure 1B). Autism patients have elevated inflammatory activity and abnormal oxidative stress which make them susceptible to short telomeres [30]. Telomere length shortening is a remarkable biomarker for other neurodegenerative diseases like Alzheimer disease confirming the role of environmental factors and chronic stress caused in inducing age related as well as neurodegenerative diseases [31-33]. We reported no significant difference in RTL between males and females in the control group (Table 3). Considering the hypothesis that females possess a more efficient antioxidant defense mechanism more than males and consequently a longer RTL than males. A reasonable explanation for our result that detecting gender effect on telomere length requires a larger number of subjects of significantly older age on other hand we noted that previous studies performed on a small group of subjects and relatively young individual (or children) like our study have been failed to detect gender effect on RTL [13,30,34-38].
Moreover, we reported a significantly low level of total glutathione (tGSH, GSH/GSSG) concentrations in autistics (Figure 2A) [39,40]. Glutathione is the major antioxidant in nearly all mammalian tissue types, with an exceptionally abundant concentration in liver tissues due to detoxification processes [15]. And it is necessary for the neutralizing ofreactive oxygen species (or free radicals), as well as the regeneration of other antioxidants such as vitamins C and E [40]. In the brain, GSH is a key determinant of cell signaling, cell proliferation, cell differentiation, protein function, apoptosis, immune function, fibrogenesis, and gene expression [40,41]. Reported that glutathione plays an important role in the brain as an antioxidant, however, it has been found that the GSH levels are the same in the brain of adult men with and without ASD, using magnetic resonance spectroscopy (MRS) [42]. Moreover glutathione reductase level in blood was the same in control and autistics [39]. In the control group, we found that males have slightly more glutathione levels than females (p = 0.026). It was previously reported that females have lower GSH levels and higher GSSG levels than males [43]. Females have more level of GPx (glutathione peroxidase), GPx is important for oxidizing GSH into GSSG as well as for catabolizing organic hydroperoxides and H 2 O2 [44]. Accordingly, females’ mitochondria produce significantly less hydrogen peroxide than males [45,46]. Interestingly, taking the hypothesis that oxidative stress precedes and causing autism by inducing telomere shortening and DNA damage, this could be an additional explanation for why the majority of autistics are males (70% males and 30 % females) [30,47]. In the autistics group, we didn’t report any correlation between gender identity and tGSH level (Table 3).
tGSH had no significant correlation with the severity of autism or with the patient clinical features including CARS, ADI-R scores, weight, height, and head circumference. This may agree with the hypothesis that oxidative stress is preceding and causing autism. While we reported an inverse correlation between RTL and ADI-R 1st domain (qualitative abnormalities in reciprocal social interaction. (r = - 0.354, P = 0.037). A correlation between telomere length and sensory symptoms of autism reported in previous studies [12]. Taking the hypothesis that chronic oxidative stress can induce telomere shortening and DNA damage the leading to autism progression [48].
Regardless of the previously reported inverse correlation between age and either tGSH or RTL in normal individuals, we found no correlation between age and neither tGSH nor RTL in the autistic group [49,50]. The corresponding p-values for the correlation between either RTL or (tGSH) and age were (P = 0.22 & P = 0.447) respectively (Table 4&5) (Figure 3 A&B). Interestingly, several studies including us reported no correlation between age and oxidative stress markers (RTL & tGSH) among affected groups (e.g. neurodegeneration, cardiovascular and diabetes) [30,51,52]. Here we hypothesis that oxidative stress in autistic is masking and dominate the age-related oxidative stress, so we were no longer able to detect age effect inactive groups (autistic). We can also consider the technical difficulty in recording the variations among critically short RTL or low level of tGSH that human body maintains to avoid apoptotic e.g. (ferroptotic) [53,54]. On other hand, chronic exposure to some toxins and pollution is producing a significant effect on glutathione level and was detectable among autistics [40].
We evaluated tGSH and RTL as a possible biomarker for autism using receiver operating characteristics (ROC) curves. A cut off for RTL at ≤ 0.72 is giving sensitivity of 72.5%, specificity of 65%. While in tGSH level a cut off value at ≤ 7.1 producing sensitivity of 80%, and specificity was 75%. We found that tGSH and RTL are a potential biomarker for autism, and maybe recommended for future studies on evaluating the effect of dietary adequacy on oxidative stress as well as improving autism associated symptoms [55,56].
In conclusion it was highly mandated to analyze the extent of oxidative stress in autistic children who lives in a developing country like Egypt. Where, the high rate of consanguinity and daily life burden that inducing additional stress. We found that oxidative stress associated to autism has a significant effect on telomere length and glutathione level among autistic Egyptian children. We found no correlation between tGSH levels and autism symptoms. Nevertheless the RTL showed an inverse correlation with some autism symptoms. This finding supports the hypothesis that oxidative stress is preceding and causing autism by affecting the integrity of human genome including telomeres. ROC analysis confirmed the higher sensitivity and specificity between tGSH and autism. Finally we are suggesting both RTL & tGSH as potential biomarkers for autism in early childhood.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval: The whole study is approved by the Ethics Committee of National Research Centre, Giza, Egypt.
Acknowledgements: we are grateful to the Autism Spectrum Disorders Clinic, Medical Research Center of Excellence, at the National Research Centre for their support.
Conflicts of Interest: The authors declare that they have no competing interests.
