Author(s): <p>Lara Santana Rodrigues, Ana Luiza Nogueira de Sousa, Mariana Xavier da Silveira Furlan, Mariana Rodrigues Borgomoni, Jeniffer Colman Pacifico, Letícia Rocha Damasceno and João Kleber de Almeida Gentile*</p>
This literature review aims to analyze the surgical treatment of enteroatmospheric fistula (EAF) and its effectiveness. Based on studies carried out from 2013 to 2022 in the PubMed, Google Academic, and Medline databases to analyze the surgical technique of the case report and compare it with other literature. The enteroatmospheric fistula (EAF), as its name indicates, is defined as an opening that connects the gastrointestinal tract and the atmosphere, located in an open abdomen, being a consequence of abdominal surgeries, traumas, perforations, ischemia, and leakage of the anastomosis after surgery. The amount of time the abdomen stays open is directly related to the risk of EAF. That is, the longer the time with the abdomen open, the more significant the risk of EAF. Among the types of fistulas, those with a high volume (>500mL) are considered the most lethal. Spontaneous closure is complex and rare, as there is not an abundance of vascularized tissue in the region, in addition to the fact that the tissue is in constant contact with the contents of the gastrointestinal tract, causing tissue irritation and delaying healing speed. Surgical procedures may be necessary.
Laparostomy is a surgical technique in which the abdomen remains open. It treats intra-abdominal sepsis, peritonitis, intra-abdominal hypertension, abdominal compartment syndrome, abdominal aortic artery rupture, and acute ischemia, among others [1-6]. The patient subjected to this surgery must remain in the intensive care unit (ICU) to monitor and observe possible complications such as electrolyte disorders and dehydration from contact with external air, sepsis, and enteroatmospheric fistulae, which this article will focus on [2].
Enteroatmospheric fistulae (EAF), as the name suggests, defined as an abdominal opening that connects the gastrointestinal tract to the atmosphere, occurs as a result of an open abdomen caused by abdominal surgery, trauma, perforation, ischemia and anastomosis leakage after surgery [7,8].
The gastrointestinal content comes into contact with the abdominal cavity, causing viscera exposure to microorganisms, irritation of mucous membranes, hydroelectrolytic disorders, fluid loss, malnutrition, unpleasant odor, and sepsis [2,8,9]. Approximately 25% of laparostomies cause EAF [10]. According to Marinis et al. 2, EAF’s incidence depends on the cause of the open abdomen, with up to 25% being from patients who suffered trauma, 25% for patients with abdominal sepsis, and up to 50% of cases of pancreatic necrosis with infection. The mortality rate varies among studies; some report 6% to 30%, and others indicate it to be higher than 64% [7,11].
EAF can be classified as follows [2,7]:
It is related that the longer the time with an open abdomen, the greater the risk of EAF 8, 7, with the higher volume (>500 mL) being the most lethal [7,8] 7. Spontaneous closure is complex and rare since there is not enough vascularized tissue and the region’s tissue is in constant contact with the contents of the gastrointestinal tract, resulting in irritation and slowing down the healing’s speed, requiring surgical treatment [2,5,8,13].
The EAF, as mentioned, frequently originates from surgical reexploration, complex abdominal traumas, resection of the colon, and, sometimes, the rectum, anastomotic fistulas, and intestinal perforation 7. Fistulas usually happen in those situations because, with the abdominal incision, there is lateral displacement of the rectus muscles. In addition, there is lateral traction by the external and internal oblique, also transverse muscle [11].
In the open abdomen, adhesions are formed between the intestine’s parts and between the intestines and abdominal wall, forming the granulation tissue in the regions lining the intestinal wall [11].
Granulation tissue is very fragile, specially in the beginning of the process. Therefore, any traction risks breaking layers of the intestinal wall and peritoneum. Some common pulling factors such as tension, coughing, limb movements, or even a BMI greater than 30 may be enough to generate a risk of tearing [11].
Around the fistula, the skin is not uniform, and the viscera are constantly exposed to the gastrointestinal contents, causing irritation in the region and growth of bacteria, making the site prone to delayed healing and greater chances of bacteremia and sepsis [11].
The sudden appearance of an enteroatmospheric fistula (EAF) after any abdominal surgery is challenging for both the surgeon and the patient. Occasionally, it is a condition associated with hydroelectrolytic disorders, acid-base disturbances as well as nutritional deficiencies which result from the hypercatabolic state (hypoalbuminemia and hyperproteinemia), severe dehydration, which is consequent to excessive fluid losses from the exposed OA surface, and septic wound complications, due to extravasation of enteric effluents into the open abdominal surface [2,9,13].
An enteroatmospheric fistula can cause several distressing symptoms in the patient. For example, patients with high-output fistula can develop hydroelectrolytic imbalances, which leads to massive fluid loss. If not treated properly, this fluid loss can lead to skin erosion and physical discomfort for the patient. If the patient keeps losing a high amount of fluids, they can develop malnutrition, which contributes directly to low protein levels and edema. As a result, these patients will need nutritional support [8].
Enteroatmospheric fistulas have specific characteristics which define them, such as the absence of an actual fistula tract, lack of a well-vascularized tissue, and its location, within an open abdomen. All of these factors contribute to the extravasation of enteric contents directly into the open peritoneal cavity, which results in complications in the septic wound. Furthermore, it reduces the probability of spontaneous closure [2,13,14].
Patients with an open abdomen who have developed EAF typically are in a critical and hypercatabolic state, which leads to a fast deterioration of their clinical status. Unfortunately, achieving a proximal detour of the enteric contents is technically impossible because of the intestinal edema, thickened and shortened mesentery, and the noncompliant abdominal wall [2].
Enteroatmospheric fistulas are categorized according to their anatomic location (proximal/distal), the volume of the fistula’s output (low < 200 mL / moderate 200-500 mL / high > 500 mL), location in the open abdomen (superficial/deep) and the number of fistulas (single, multiple proximal fistulas or multiple distal fistulas). Some of these parameters affect prognosis and spontaneous closure rate, for example, the anatomic location and the volume output. In other words, distal and low-output EAFs have a high spontaneous closure rate compared to more proximal and high output EAFs. Although superficial EAFs are more common in clinical practice, they are more difficult to manipulate due to the difficulty of effectively controlling enteric spillage at the open abdomen surface, which can trigger a septic condition [2].
Various diagnostic methods can demonstrate the fistula location and intra-abdominal abscesses and exclude distal intestinal obstruction. Some of these are the methylene blue test, fistulography, computed tomography, magnetic resonance imaging, and oral or nasogastric ingestion of charcoal or dye [2,15].
The treatment of enteroatmospheric fistulae (EAF) has a clear goal: to limit and control its acute phase [13] 13. The correct management involves monitoring, supporting treatment, and EAF’s closure, which can happen spontaneously or through definitive surgery [2,16] 2, 16. Theoretically, a valid alternative to contain the infection would be an urgent laparotomy; however, it is not a viable option due to intestinal edema and dense adhesions, which make this procedure impossible [13,15]. Therefore, the fistula must be isolated, and a dressing must be applied to allow primary closure [15]. When it is impossible to close the fistula, the medical conduct should aim to allow the acute fistula to become chronic and controlled [13].
With the correct management, there is a slight chance of a fistula’s spontaneous closure9. However, the recurrence is more likely if compared to those surgically closed [2,15]. In most cases, spontaneous closure is impossible due to the insufficient amount of vascularized overlying tissue, the efflux of irritating content, and the continuous exposure of the intestine, which leads to protein loss and sepsis [9]. Hence, this closure pattern is more probable to occur in small EAFs with low output, thus, making the surgical treatment necessary in most cases [15].
If the fistula does not close spontaneously in 6 weeks, surgical treatment is mandatory; nonetheless, the required waiting period is 6 to 12 months, so the abdomen gets to proper conditions before surgery. During these months, the intestinal adhesions become more malleable. The inflammatory process is reduced, diminishing the risk of intestinal lesions. If the EAF is surgically closed before the abdomen reaches proper conditions, it can favor the occurrence of complications, such as EAF recurrence, intestinal injury, sepsis, and even death [15]. This surgery can only be performed in wellnourished and physiologically stable patients [13]. This operation consists of resectioning the intestine’s fistulized part, posterior restoration of its continuity, and coverage with well-perfused tissue [15].
The proper support treatment must aspire to control the source of infection, prevention or recognition, and early treatment of sepsis, thus trying to avoid the progression of the patient’s state to generalized organ failure. This treatment must also correct electrolyte and acid-base disturbances and revert the hypercatabolic state [2,13,15]. It is crucial to collect, quantify, and characterize the fistula’s output since those aspects change the EAF’s prognosis. Fistula’s secretion will also directly influence the choice of antibiotic in case of sepsis since it is culture material. Moreover, it should reduce EAF’s spillage of enteric content through the use of proton pump inhibitors and somatostatin or its analogous; and prevent malnourishment, which could worsen the patient’s condition, by initiating parenteral nutrition [13].
Other measures should be taken, such as protecting the exposed organ and its surrounding tissues and deviating the course of EAF’s secretion, making it possible for the exposed bowel tissue to become granulation tissue. To achieve this is necessary to apply a temporary abdominal closure (TAC) technique [13,15]. There are many types of TAC, and they all fit into two categories: passive visceral coverage and negative pressure (NP) techniques. Negative pressure techniques have the advantages of maintaining the abdominal wall integrity and draining the intraperitoneal fluid. Several methods to apply NP exist, but the most common is the Barker vacuum-packing technique (BVPT) because of its simplicity, low cost, and the availability of its materials in most operating rooms (OR) [17].
BVPT has four layers. The first one consists of a perforated polyethylene sheet, a non-adherent material, set underneath the peritoneum of the abdominal wall to protect the viscera and prevent adhesions of the bowel to itself and the abdominal wall. This layer also avoids the constant reexploration of the abdominal cavity, allows the abdominal wall to move towards the midline and the passage of fluids through the perforations to the vacuum system [16].
BVPT’s second layer builds on suction drains and compressible material, like macroporous polyurethane foam or surgical towels, in contact with the fascia and its edges between the parietal peritoneum and the first layer. Surgeons may use skin staples to bring the skin edges closer and keep the foam in place. This layer prevents the intestine from protruding through the open abdominal wall and prevents fascial retraction because when negative pressure is applied, it becomes semirigid, creating medial facial tension even without a suture [16].
The third layer of the dressing comprises silicon drains on top of the second layer, connected to a negative pressure system that maintains the pressure between 100 and 150 mmHg. Their function is to control the efflux of intra-abdominal fluid (Figure 1) [16].
The fourth and last layer protects the skin adjacent to the open part of the abdomen and completes the vacuum seal. This layer is an adhesive sheet. Once sealed the dressing and pressure applied to it, it must remain intact until reexploration is possible, and in this time, the contraction of the wound can be perceived (Figure 2) [16].
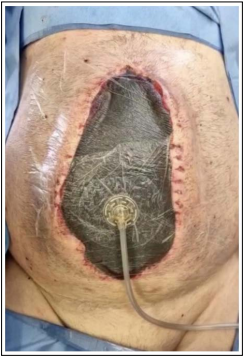
Figure 1: Barker vacuum-packing technique (BVPT) connected to the negative pressure system switched off. (author’s archive)
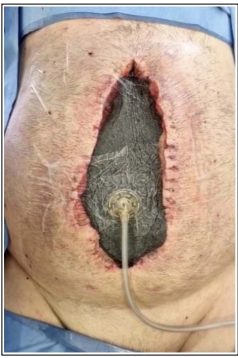
Figure 2: Barker vacuum-packing technique (BVPT) connected to the negative pressure system with pressure applied. The wound’s contraction is easily perceived. (author’s archive)
The enteroatmospheric fistula (EAF) is a consequence of an open abdomen. It has three types of classifications, and they directly imply the severity of the consequences that the patient will suffer. Furthermore, treating and controlling its acute phase is essential to keep the patient from progressing to an unfavorable prognosis. A definitive surgical closure will be necessary if spontaneous closure does not occur, and in the meantime, a TAC application is necessary. There are multiple methods to apply TAC. The most common type, the BVPT, consists of four layers compounded by polyethylene foam, suction drains, a negative pressure device, and an adhesive bandage, because of its material availability and low cost.
Research Ethics Committee Approval:
We declare that the patient approved the study by signing an informed consent form and the study followed the ethical guidelines established by the Declaration of Helsinki.
Conflict of Interest: We Have No Conflict of Interest
Acknowledgements: For All Researchers of This Study
Funding: We Have no Funding Support
