Author(s): Girish J Kotwal
COVID has become one of the deadliest obligate intracellular pathogens in over 100 years. COVID pandemic’s devastation causing to date 6.05+ million global deaths from over 460 million confirmed positive infections globally in just 2 years and close to 964,000+ US deaths out of 79.5 million confirmed positive cases in the US, continues with no sign of eradication of the virus. Antigenic variation is a predominant way of microorganisms causing human diseases to evade innate and acquired immunity. A major reason for that is COVID’s ability to evade human innate and acquired immune defenses by antigenic variation and to breach the weaker immune defenses of those among us who could not mount a timely and effective immune response to sustain ferocious attack on lungs and survive with great difficulty or succumb to an inability to breathe. While it is globally observed by Physician scientists that the OMICRON variant is highly transmissible but causes only MILD symptoms, because it is present only in the upper respiratory tract. A more virulent variant having a similar ability to spread fast could be catastrophic. How can immune evasion by COVID and its emerging variants be circumvented with better vaccines and treatments? It is high time to develop second generation of vaccines with broader protection that more closely simulates natural infection and viral entry inhibitors, eg. enveloped virus neutralizing compounds (EVNCs) that have proven broad spectrum neutralization of pandemic Influenza viruses like H5N1 or SARS-CoV-1, could prove effective against emerged and rapidly emerging strains of COVID like Omicron. A lab. Report a year after prior severe COVID infections but without any vaccine or booster dose clearly suggests that natural immunity acquired during infection is long lasting and should be meaningfully recognized by CDC and other agencies as, at least equivalent by immunization with vaccines against the emerging variants mutating periodically due to genetic variation and viral evasion.
As 5 new variants have emerged in a span of 2 years, from the original COVID that escaped from Wuhan, China and made a home in every nook and corner of our planet, it is clear to those of us who study how viruses evade innate and adaptive human immune response, as to how evasion of human defenses has likely made emerging variants like OMICRON more transmissible to an extent that in the USA, 95% of current COVID cases are due to OMICRON. We have known for decades, DNA viruses (eg. Poxviruses) after multiple passaging in cell culture Or RNA viruses passaged in human hosts accumulate mutations spontaneously, some of which may make a virus more transmissible or even less virulent Corona [1-3]. Viruses replicate rapidly, inside host cells when its RNA polymerase duplicates its genomic RNA, without an ability to prevent multiple mutations. Many mutations may hinder its ability to replicate but a few could change its invasive and immune evasion capacity to make it more transmissible or even make it deadlier than the wildtype virus. Omicron variant and the delta variant are prime examples of emergence of variants endowed with new characteristics that become public health crises.
According to the CDC, most reported U.S. Omicron cases are detected in the fully vaccinated although with MILD symptoms and the few who were previously infected and acquired immunity have MILDER symptoms [4]. Is it possible that Omicron emerged as variant after 50+ mutations in the genomic RNA of COVID so that it could spread most efficiently across the vaccinated global population by evading neutralizing antibodies against the Spike protein of OMICRON? In fact, South African scientists discovered Omicron after a peak in COVID cases in the vaccinated population [5-8]. Omicron confirmed what virologists feared that sooner rather than later a variant will emerge that will successfully evade the immune response elicited by the currently FDA approved vaccines against COVID and OMICRON could be first such variant of the many variants [9]. That have emerged since the beginning of the pandemic 2 years ago and IHU that has justemerged in France could be another [10].
Some virologists were therefore advocating for a new generation of vaccines and natural antivirals to human enveloped viruses that would overcome immune evasion of vaccine elicited immunity by not exclusively targeting the spike protein amino acid backbone of COVID elicited immunity, but also serve as broad spectrum entry inhibiting antivirals against the glycosyl moieties of surface glycoproteins [11-15]. The optimal design of future vaccines would be to develop vaccines that will more closely simulate longer lasting superiorly broad B and T cell immunity against not just the spike surface glycoprotein of COVID but also other proteins like the most abundant nucleocapsid protein inside the COVID virion [16]. A close examination of the 50+mutations reveal that 30 of the mutations found in the spike protein and predominantly in the receptor binding site of OMICRON spike protein (Table 1) and only 4 mutations in nucleocapsid protein and out of the 4, only 2 are functionally significant because amino acid changes possibly explain the increased viral load and explain higher transmissibility of OMICRON. Higher transmissibility can also be explained by some of the mutations in the spike protein and developing second-generation mRNA vaccine that will encapsulate in the nanoliposomes, the precise RNA encoding OMICRON RNA sequence for spike protein as well as the RNA encoding nucleocapsid protein will ensure better protection against the fast-spreading OMICRON variant.

Microorganisms which evolved to become successful pathogens are all endowed with an ability to evade the innate and adaptive immunity of the host. The best-characterized parasites that undergo rapid hyperactive mutation to cause antigenic variation are trypanosomes, and viruses like Influenza, HIV and now Coronaviruses, SARS-CoV-2 and its variants (Table 2) [4,17]. A unifying theme of antigenic variation is the changes in the amino acid protein backbone of surface antigens, which allows for escaping neutralizing antibody responses by staying one-step ahead of the host’s immune response chasing the parasite by developing a response against constantly changing antigens of the parasite.
The tightly regulated powerful complement system consisting of 30 circulating and surface receptor proteins is the first line ofdefense against microorganism surfaces recognized based on their unique surface pattern as foreign by circulating complement components [18]. There are 3 major pathways of complement activation, classical, alternate or alternative and the lectin pathway. All 3 pathways can result in the formation of the C3 convertase which can then form the C5 convertase and which in turn can form the membrane attack complex (MAC) which culminates into lysis of virus infected cells or the virus itself or a unicellular microbe. Along the pathway chemotactic factors, C3a, C4a, C5a and MAC can elicit an inflammatory response which can cause phagocytosis of the viruses and microbes. Proteins like C3b and iC3b can target microbes for opsonization by macrophages with receptors for these molecules. The best characterized active evasion mechanism was studied in poxviruses and has been subsequently studied in several other viruses and self-replicating pathogens [2]. Large DNA viruses like poxviruses and Herpes viruses encode proteins not only to support their multiplication but also to specifically evade host defense. One of the first microbial encoded complement protein with structural similarity to human and mammalian complement control protein to be discovered was the vaccinia virus complement protein from vaccinia virus [19]. It was later shown to inhibit the classical complement pathway [20]. Soon it became clear that in order to, overcome the first line of defense, a pathogen has to have some way to evade the complement onslaught, but RNA viruses with a limited genomic capacity had to have other more subtle ways of evading complement and it was found that HIV and other viruses insert in their membrane, cellular complement receptors while exiting from the cells as a way to resist complement mediated neutralization [21]. It is unclear at the current time how exactly Coronaviruses evade host complement response, but it is evident that the host response elicited to COVID presence is quite ferocious and thought to cause complications like clotting in critical organs which initially caused a lot of deaths. Subsequently anti-clotting agents like heparin and lovenox have become standard of optimal care. There are currently other complement inhibitors considered as potential treatment of elevated complement levels in patients developing hyper immunity or clotting disorders [22]. Cytokines especially interferons are well known for their antiviral activities and therefore like complement inhibition; successful viral pathogens have active mechanisms mediated by viral encoded proteins to evade host antiviral effects. Evidence that antiviral effects of Interferon II and I are counteracted by COVID encoded proteins COVID has been clearly demonstrated and are summarized in Table 2 [23,24].
| Passive evasion by Antigenic variations of Omicron | ||
|---|---|---|
| COVID Protein | # of amino acid mutations. | References |
| Spike protein | 30 | Martin et al., 2021Goldbold, 2021 |
| Nucleocapsid protein | 4 | Hodcroft et al., 2022Wang and Cheng, 2021 |
| COVID proteins. | Active evasion of Cytokine Secretion | References |
| N, M, | IFN-1 and IFN-III. | Kim and Shin, 2021Honjie et al., 2020 |
| NSPs ( 1, 3b,4a,4b, 5, 6), PLpro | Taefehshokr et. al, 2020 | |
| Immune cells. | Aggressive Evasion mechanism. | References |
| NK cells | NK cell exhaustion. | Taefehshokr et. al, 2020 |
| Neutrophils | Direct entry via ACE-2 receptor. | Taefehshokr et. al, 2020 |
| Macrophages | Direct entry via ACE-2 receptor. | Taefehshokr et. al, 2020 |
| Dendritic cells | Direct entry and Il-12 secretion. | Taefehshokr et. al, 2020 |
COVID and its variants have been shown to have an ability to enter a wide range of human cells in addition to the cells in lungs. Entry into immune cells by viruses is an aggressive way to destroy the very cells that are key to mounting an immune response to pathogens (table 2). Omicron has evolved to be less virulent because it mainly remains restricted to the upper respiratory tract and so far reports are that OMICRON symptoms are MILD unlike the alpha to delta variants and that is primarily because there is no lung damage therefore not requiring breathing assistance for severe acute respiratory syndrome (SARS). ACE2 receptors are present in several organs including in the brain [25].This allows for aggressive neuroinvasion and a place to escape circulating immune surveillance by large molecules and peripheral immune cells restricted from entry past the blood brain barrier [26]. Despite that COVID and its variants have been detected in brains associated with causing wide-ranging symptoms from brain fog to tinnitus, if sub-optimally treated with the current antivirals or if treatment is delayed.
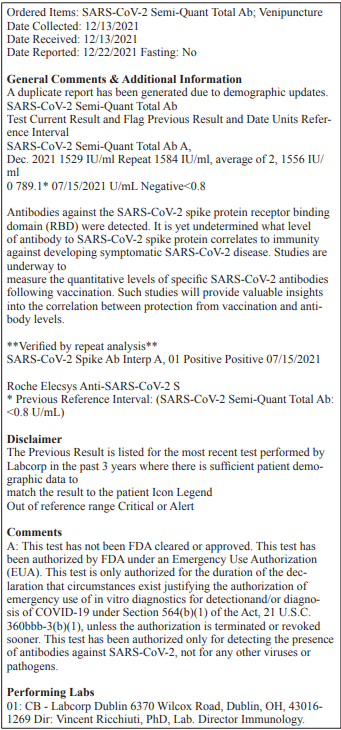
The rapid emergence of COVID variants ranging from alpha to OMICRON despite vaccines and antivirals is alarming [27-28]. Two years since the pandemic spread to every nook and corner of the world, COVID’s continuing antigenic variation have resulted in emergence of variants with mutations that allow for efficiently escaping human immune repertoire of antibodies. Omicron has proved to be the ultimate immune escape artist because it escapes immunity acquired from vaccines even though it has so far been found to be highly transmissible, less virulent, and not as invasive. Boosters seem to be reported to be effective against OMICRON but there is still concern as to why fully vaccinated individuals do not have sufficient memory cells to generate the neutralizing antibodies that were effective previously. Again, a close examination of the amino acid changes within the antigenic sites in OMICRON suggest that the antibodies elicited from earlier administration of the vaccines may have a reduced affinity to the antigenic sites of OMICRON or that the antibody levels fall sharply requiring boosting. It is time for developing second generation vaccines that can circumvent antigenic variation of spike proteins of emerging variants by including mRNA encoding both a combination of spike proteins for specific variants andmore conserved nucleocapsid protein inside the nanoliposomes formulation injected as vaccine. This will simulate an actual infection more closely, which not only has antibodies to the spike protein but also the major protein, the nucleocapsid protein, which will provide a broader immunity in real time as opposed to without anticipation of what immunity, the vaccine will actually, elicit, that a variant will evade. As COVID variants passage through more millions of human hosts, the rate at which variants are emerging (1 variant per 60 million infections) all around the world will be difficult to control and hence a strategic approach is in order.
Broad spectrum natural antivirals such as the polyphenols from pomegranate or carbohydrate derived fulvic acid from natural humic substances and shilajit material [12,13, 29, 30, 31] that neutralize enveloped viruses by binding to the host derived glycosyl chains on specific amino acid glycosylation sites like Aspargine-X-Serine/Threonine (X being any amino acid, but not proline) on the surface glycoprotein could be explored [13,29]. No matter what mutations occur in the protein that give rise to variants; because COVID spike protein is heavily glycosylated and transfer of the assembled sugar chains during biosynthesis of the membrane embedded glycoproteins is a host function, targeting conserved glycosylation sites unlikely to be liable to the vagaries of mutations would be a more secure way of neutralizing extracellular virus in tissues [30, 31].
In 2 years since the WHO declared SARS-COV-2/COVID a pandemic, there have been 5 variants of concern. That is roughly 2-3 variants per year all due to mutations mostly in the polypeptide backbone of the spike surface glycoprotein. All mutations can be attributed to natural passage among humans with an ability to transform to acquisition of new functions, either increased pathogenicity or increased transmissibility or increased ability to evade the neutralization by host antibodies due to surface antigenic variation in the virus. The worst case, scenario will be if a variant emerges that will have the lethality of delta, the transmissibility of OMICRON and evasion of immunity acquired from all current vaccines that develop immunity exclusively to the spike protein. Should that happen, we will be back to square one, similar, to as in 2020, when there were no safe and effective vaccines, and the death rate was very high. At the end of 2 years, we are beginning to realize there is no substitute to broad, long lasting protective immunity a year later following clearance of COVID infection (Table 3), similar, to that acquired following a severe COVID infection [16]. Therefore, it is time to develop vaccines that will generate antibodies not only to the hyper mutating surface glycoprotein, the spike protein but also to the conserved nucleocapsid protein, a major protein of SAR-CoV-2 and its subsequent variants. Nucleocapsid proteins of other viruses like influenza have been shown to elicit anti-viral infected cell, cytotoxic T cell response. Just as there is a seasonal flu vaccine every winter in the northern and southern hemisphere based on the WHO committee deciding on which Inactivated flu strains to include in a vaccine for a particular season, we need for the WHO to decide which mRNAs to encapsulate in the vaccine nanoliposome formulation. Alternatively, a live attenuated vaccine could be developed along the lines of Omicron sequence but significantly more attenuated. Neutralizing monoclonal antibodies as antivirals could also be developed to neutralize new variants, as it is clear, that many of the current ones that were effective against delta are going to be soon ineffective against newer variants as it is become increasing clear that mutations in Omicron spike (Table 1) are diminishing the ability of the monoclonals to bind. As far as a new generation of entry inhibiting safe antivirals, broad spectrum enveloped virus-neutralizing agents will overcome rapid antigenic variation and could provide stability from the vagaries of the unpredictability of the mutations that could result in evasion of neutralizing antibodies.
Review of current state in the field of evasion of human innate and acquired immunity by SARS-CoV-2/COVID variant OMICRON.
