Author(s): <p>Mukur Dipi Ray, Amitabha Mandal<sup>*</sup>, Sudhakar Gunasekar, Vinod Kumar</p>
Introduction: Hyperthermic intraperitoneal chemotherapy (HIPEC) is a well-known procedure for peritoneal surface malignancies. Usually, the procedure is supposed to be performed in the same setting after completion of optimal cytoreductive surgery (CRS). Due to some intraoperative issues, we performed HIPEC in early postoperative period or else around 30 days of index surgery. We call this protocol as “Staged HIPEC”.
Material & Methods: Fourteen patients were included from September 2017 to November 2019 from a prospectively maintained database. Patients in whom HIPEC procedure was deferred in the same sitting following optimal CRS, due to various reasons & it was performed either in the early postoperative period (1-3 days) or around 30 days (4 – 6 weeks) of index surgery, were included in this study. All the patients were followed up till 31st March 2020.
Results: During CRS twelve patients (85.7%) developed hypotension with oliguria and two patients (14.2%) developed tachyarrhythmia. Clavin Dindo grade II & grade III complication occurred in 7 patients (50%) & five patients (35.7%) respectively. No complication occurred due to Staged HIPEC. No perioperative mortality was observed in the current study. Average disease-free interval was 13.5 months (range 4-21 months).
Conclusion: As there were no significant complications occurred due to staged HIPEC and the disease-free interval was comparable to CRS & HIPEC procedure in the same setting, “staged HIPEC” may be a feasible option for those patients, who were hemodynamically unstable during CRS, and HIPEC procedure, was not possible at the same setting.
Hyperthermic intraperitoneal chemotherapy (HIPEC) is a well-known procedure for peritoneal surface malignancies like pseudomyxoma peritonei (PMP), mucinous neoplasm of the appendix, mesothelioma, and in a subset of advanced epithelial ovarian and colonic malignancies [1-4]. The most common indication of HIPEC is mesothelioma, followed by PMP [1,2]. Usually, the procedure is supposed to be performed in the same setting after completion of optimal cytoreductive surgery (CRS). The procedure is well known as CRS with HIPEC. Usually, optimal CRS requires ample time to complete the procedure, so performing HIPEC at the same sitting may not be always possible because of hypotension, low urine output, tachycardia, or when the patient is on ionotropic support. So what ideally should be done in a situation of intaroperative events which forced the surgeon to defer HIPEC procedure in the same setting of CRS? Can we perform HIPEC in the second surgery as a staged procedure, in the postoperative period? Keeping those questions in mind we performed HIPEC procedure in 14 patients in postoperative period as a staged procedure. We call this protocol “Staged HIPEC”.
Fourteen patients underwent staged HIPEC in Dr. BRAIRCH, AIIMS, New Delhi from September 2017 to November 2019. The data were collected from a prospectively maintained database of Surgical Oncology Department.
1. The main objective of this study is to evaluate the feasibility
of the “STAGED HIPEC”
2. Safety and efficacy of the procedure
3. Complications associated with the procedure
Patients were shifted to the intensive care unit (ICU) after surgery to stabilize the hemodynamic condition and planned for HIPEC within 72 hours (1-3 days), preferably on the first post-operative day or else around 30 days (4 - 6 weeks) of index surgery, after the abdomen is settled down. Staged HIPEC was performed under general anesthesia in the early postoperative period. Body surface area (BSA) was calculated for each patient preoperatively. Cisplatin was used in 13 patients at a dose of 70mg/sq m or 45 - 52 mg/ 1 L of NS. Mitomycin was used in one patient of PMP at a dose of 20mg/sq m. HIPEC was instituted using a semi-open “Coliseum technique” [Figure 1] for 60 minutes in all cases. The procedure was performed at a temperature of 41-43 degrees celsius. Normal saline was used as perfusate for all procedures at a mean volume of 2.1litres.
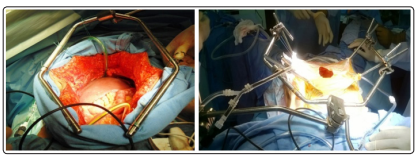
Figure 1: Semi Open Coliseum Technique for Hipec
The study is a descriptive retrospective study 0f 14 cases of “STAGED HIPEC”.
1. Surgical complications were graded as per the Clavin Dindo
grading system.
2. The disease-free interval was calculated from the treatment
completion date to the date of recurrence.
Fourteen patients underwent staged HIPEC during the study period. 9 patients of stage IIIc carcinoma ovary (primary malignancy - 4 patients & recurrent malignancy - 5 patients), 2 cases of PMP, 1 each of malignant mesothelioma, carcinoma stomach, and gastric GIST were included in this study. High-grade serous carcinoma (HGSC, 8 patients) was the most commonly encountered histology among carcinoma ovary patients, followed by low-grade papillary serous carcinoma (1 patient). Low-grade mucinous neoplasm of the appendix (LAMN) was the histology found in both the cases of PMP. The clinical profile of these patients is summarized in [table 1].
| Case | Age | Sex | ECOG PS | Diagnosis | Histology |
|---|---|---|---|---|---|
| Case 1 | 48 | F | 1 | PMP (Primary) | LAMN |
| Case 2 | 45 | M | 1 | PMP (Primary) | LAMN |
| Case 3 | 40 | F | 1 | Ca ovary (Primary) | HGSC |
| Case 4 | 51 | F | 1 | Ca ovary (Primary) | HGSC |
| Case 5 | 48 | F | 2 | Ca ovary (Recurrent) | HGSC |
| Case 6 | 46 | F | 2 | Ca ovary (Recurrent) | HGSC |
| Case 7 | 44 | F | 1 | Ca ovary (Recurrent) | HGSC |
| Case 8 | 70 | F | 1 | Ca ovary (Primary) | Serous adenocarcinoma |
| Case 9 | 25 | F | 1 | Ca ovary (Primary) | Low grade papillary serous carcinoma |
| Case 10 | 63 | F | 2 | Ca stomach (Primary) | PD adeno carcinoma |
| Case 11 | 32 | F | 1 | Peritoneal mesothelioma (Primary) | Adenocarcinoma |
| Case 12 | 50 | F | 1 | Gastric GIST (Recurrent) | GIST |
| Case 13 | 55 | F | 1 | Ca ovary (Recurrent) | HGSC |
| Case 14 | 56 | M | 1 | Ca ovary (Recurrent) | HGSC |
Staged HIPEC was performed in all fourteen patients in the early postoperative period (9 patients) or around 30 days (5 patients) of optimal CRS. The mean (BSA) was 1.51, ranging between 1.30 - 1.92. During CRS twelve patients (85.7%) developed hypotension with oliguria and two patients (14.2%) developed tachyarrhythmia. Optimal cytoreduction was achieved in all patients. Completeness of cytoreductive score (CC score) were 0 and 1 in thirteen and one patient respectively. The mean peritoneal carcinomatosis index (PCI) was 10, ranging between 1-24.
During CRS, procedures like bowel resection, bladder peritonectomy, diaphragmatic stripping, were performed in 5 patients (35.7 %), 3 patients (21.4 %), 3 patients (21.4 %) respectively. Diaphragm repair and bladder wall repair was done in one patient each (7.1%). Intraoperative ureteric injury occurred in one patient, repaired primarily.
The mean duration of surgery (CRS) was 395 minutes. For those patients who had undergone Staged HIPEC in the early postoperative period, the mean ICU stays and postoperative hospital stay was 3 days (range 2-8 days) and 12 days (range 6-26 days) respectively. Those patients who had undergone Staged HIPEC in the late postoperative period (around 30 days of index surgery), the mean hospital stay was 3 days.
Clavin Dindo grade II complication occurred in 7 patients (50%), among them one patient had GTCS, required antiepileptic drugs, and 6 patients required blood transfusion in the postoperative period. Grade III complications encountered in five patients (35.7%) [Table 2]. Immediate and late complications occurred in four & two patients respectively [Table 2]. No perioperative mortality was observed in the current study. Late complications developed in two patients in the form of entero-cutaneous fistula (ECF) & wound dehiscence [Table 2]. All 9 patients with carcinoma ovary received paclitaxel & carboplatin-based adjuvant chemotherapy. Two patients developed peritoneal recurrence, one in the right hypochondrium and another one in the pelvis. The average disease-free interval was 13.5 months ranging between 4-21 months.
| Early post operative complication | ||
|---|---|---|
| Complication | No of patient | Management |
| GTCS | 1 | Antiepileptic drug |
| Urinary leak from bladder | 1 | Bladder repair |
| Ureteric necrosis | 1 | Re exploration & ureteric re implantation |
| Gastric anastomotic dehiscence | 1 | EL & closure of gastric dehisence +FJ |
| Late complication | ||
| ECF | 1 | Re-exploration & resection of fistulous segment + anastomosis |
| Abdominal wound dehiscence | 1 | EL & secondary suturing |
Cytoreductive surgery and HIPEC has been considered as a standard of care in the management of pesudomyxoma peritonei and advanced ovarian epithelial cancer with acceptable morbidity and mortality [5,6,7].
Management of relapsed ovarian epithelial cancer should be tailored and individualized. It depends upon the prior treatment details and duration of disease or treatment-free interval and performance status of the patients [8,9]. Options include platinum or non-platinum based systemic chemotherapy, secondary cytoreductive surgery, antiangiogenic agents, poly ADP ribose polymerase (PARP) inhibitors, and maintenance therapy [10,11].
Optimal cytoreduction is one of the most powerful determinants of survival in advanced ovarian epithelial cancer [12]. Adding HIPEC with optimal interval cytoreduction improves the outcomes in terms of relapse-free survival and overall survival in advanced epithelial ovarian cancer [7]. Morbidity and mortality from HIPEC procedure in a review of one of the largest international series are reported to be 8 to 31 % and 3% respectively [13].
Studies also have reported that peritoneal carcinomatosis index, the extensiveness of cytoreductive surgery, duration of the total procedure, the extent of peritoneal resection, and the number of anastomoses are the independent risk factors for morbidity [13,14,15]. There is minimal data in the literature to recommend the role of secondary cytoreduction with or without HIPEC in relapsed ovarian epithelial cancer. To achieve optimal secondary cytoreduction in a relapsed ovarian epithelial cancer, predictive models like AGO score and MSKCC criteria have been recently developed [16,17].
Baumgartner et al reported a retrospective study of 247 patients who had undergone CRS-HIPEC for peritoneal carcinomatosis with median PCI of 14. In this study, the most common site of origin was appendix (67.2 %) followed by colorectal (20.6 %), peritoneal mesothelioma (8.9 %) and, ovary in 2 % of all cases. They had reported 60-day morbidity and mortality as per Clavin Dindo that grade 3 complications occurred in 13.4 %, most commonly intrathoracic or intraabdominal collection requiring pigtail drainage, grade 4 in 2%, and grade 5 (death) in 1.2% of cases. They concluded that the presence of symptoms; Charlson comorbidity index and prior resection status were important tool predictors of major complications [18].
Somsekhar et al reported a prospective study of twenty-six patients with recurrent ovarian epithelial cancer that major complications like bowel fistula occurred in 7.6% of cases requiring reexploration, temporary stoma, and wound-related complications in 26% of all cases with no mortality. Median PCI was 9.5 (Range: 3-19) and median hospital stay was 12 days (range: 10-42 days) [19]. A positive randomized study by Spiliotis et al concluded that adding HIPEC with the completeness of secondary cytoreduction had improved the outcomes in terms of overall survival both in platinum-sensitive and platinum-resistant relapsed ovarian epithelial cancer [20]. But another randomized trial published in NEJM showed that in patients with platinum-sensitive relapsed ovarian epithelial cancer, adding secondary cytoreductive surgery followed by chemotherapy did not improve longer overall survival than chemotherapy alone [21].
In the current literature, the role of staged HIPEC and its impact on outcomes in patients with relapsed ovarian epithelial cancer has not been reported. In our study series of fourteen patients, procedures like bowel resection in 35.7%, bladder peritonectomy in 21.4%, diaphragmatic stripping in 21.4%, diaphragm resection in 7.1%, bladder wall resection & repair in 7.1% of all cases were done to achieve optimal cytoreduction. The mean PCI of our patients was 10 (range between 1 -24).
The mean duration of the procedure was 395 minutes, comparable to most of the other studies [1-4]. Because of hemodynamic instability events during the cytoreductive procedure, HIPEC was not done in the same setting,, and staged HIPEC had been performed after stabilization.
One patient intraoperatively had a ureteric injury and repaired primarily. Grade 3 complication occurred in 5 cases (35.7% of cases, one had small bowel fistula, two had urine leak, one had abdominal burst wound and one had a gastric leak) requiring re-exploration under general anesthesia and grade 2 complication occurred in seven patients (one had GCTS, 6 patients received post-op blood transfusion, 50% of cases). Despite having higher morbidity, there was no mortality in our series. Staged HIPEC procedure yields minimal effect on surgical morbidities. Higher morbidity could be directly attributed to the disease burden and extensive nature of the surgery like bowel and bladder resection. Mean hospital stay in our series was 12 days (range between 6 to 26 days). The average follow-up period was 13.5 months, ranging from 4-21 months. Two patients had developed peritoneal recurrence, one in the right hypochondrium and another one in the pelvis. The average diseasefree interval was 13.5 months ranging between 4-21 months.
As there were no significant complications occurred due to staged HIPEC and the disease-free interval was comparable to CRS & HIPEC procedure in the same setting, “staged HIPEC” may be a feasible option for those patients, who were hemodynamically unstable during and after CRS, and HIPEC procedure, was not possible at the same setting. A prospective study with large sample size is required to assess the benefit of “staged HIPEC”, but our observational study suggested that “staged HIPEC” is quite feasible when it is not possible in the same setting
Acknowledgments: We acknowledge Dr. Babul Bansal ( MS, Mch Surgical Oncology, Dr. BRAIRCH, AIIMS) for his contribution in editing this manuscript.
Conflict of Interest: None
