Author(s): Jennifer Garay Guerrero
A 52-year-old woman with Raynaud syndrome diagnosed less than 1 year ago, consulted the emergency room due to 4 months of progressive symmetrical edema in the lower limbs, muscle weakness, dyspnea, hypertension crisis, and dark urine. Investigations revealed left ventricular dysfunction (ejection fraction 14%) without coronary artery disease and acute renal injury. Mixed Connective Tissue Disease was diagnosed and because of the renal and systemic findings in the patient a renal biopsy was performed confirming scleroderma renal crisis. To the best of our knowledge, this is the tenth patient who reported scleroderma renal crisis as a complication in patients with Mixed Connective Tissue Disease, from these 4 patients who became hemodialysis dependent, 1 died, and 4 responded to therapy. The treatment chosen was Angiotensin-converting-enzyme inhibitors and steroids based on what is known in renal scleroderma crisis with a substantial recovery of the left ventricular function and stabilization of the glomerular filtration rate followed by discharge from hospitalization.
Mixed Connective Tissue Disease (MCTD) was the first overlap syndrome with a specific autoantibody ever described and is characterized by high titers of serum anti-ribonucleoprotein (anti-U1 RNP) plus clinical characteristics of systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, and polymyositis. The most common symptoms include Raynaud’s phenomenon (90%), hand edema, arthritis, inflammatory muscle disease, and sclerodactyly. Other findings include high antinuclear antibodies, and hematologic abnormalities like anemia, leukopenia, lymphopenia, and thrombocytopenia. Therefore, can compromise the functioning of all the organ systems, but kidney injury is rare [1].
Phenotype is stable in most patients and durable remission is infrequent, although has a good response to immunosuppressive treatment, so, the outcome is favorable in terms of declining disease activity over time [2]. The incidence and prevalence are low, 1,9-3,3 cases per 100000 individuals, so is an uncommon disease [3]. Scleroderma renal crisis (SRC) has been described in the literature in patients diagnosed with MCTD in a few clinical reports, and when it is present is a serious complication that requires to be confirmed by renal biopsy, although is not routinely done [4].
Herein, we report a patient with a clinical history of Raynaud´s Syndrome with severe acute heart failure, acute kidney failure, hypertension emergency, and muscle weakness that during the hospitalization was diagnosed with MCTD and SRC.
A 52-year-old woman with a history of Raynaud’s Syndrome diagnosed a year ago, had been treated with amlodipine (5 mg/ day) and aspirin (100 mg/day), started with 4 months of pain in the lower left limb, a skin ulcer which healed rapidly followed by a distal color change and one-block claudication. 15 days before emergency room (ER) consultation began with symmetrical edema in the lower limbs of sudden onset, muscle weakness, and dark urine. 3 days prior to admission the patient also manifested dyspnea at rest, general malaise, and abdominal distention.
Due to worsening symptoms, she decided to consult and was admitted to the ER with cyanosis, hypoxemia, tachycardia, hypertensive encephalopathy, signs of respiratory distress, required tracheal intubation, and was transferred to the intensive care unit (ICU). Initially was suspected SARS-CoV-2 infection versus pulmonary thromboembolism (PTE). Computed Tomography angiography of the chest and abdomen was performed and ruled out PTE, but reported cardiomegaly, bilateral pleural effusion predominantly right, and incidental mature teratoma subsequently, the echocardiogram reported severe heart failure with left ventricular function (LVF) in 14%, the coronary disease was considered, but Coronary angiography discarded any important coronary lesion. In the ICU, there was evidence of Raynaud’s phenomenon in the upper limbs, and stiff skin in the lower limbs.
One week after was transferred to regular hospitalization and developed acute kidney injury KDIGO 2 (creatinine 2.03 mg/ dL), and laboratory test showed mild 24-hour proteinuria (522 mg), active urine sediment (6 red blood cells, 4 leukocytes per high-power field) and C3 hypocomplementemia (77.3 mg/ dL, normal range 90-170), normal renal ultrasonography, mild anemia (11.9 gr/dL), schistocytes on the peripheral blood smear, high lactic acid dehydrogenase (1899 U/L), positive antinuclear antibody (1:640 dilution, speckled pattern), positive nuclear ribonucleoprotein autoantibody (121 U/mL, normal range <15 U/mL) and thrombocytopenia (72× 109/L). Other laboratory tests are shown in table 1. To make a conclusive diagnosis, a renal biopsy was performed, reporting glomerular thrombotic chronic microangiopathy, arterial intimal fibro myxoid changes, and ischemic lesions (Figure 1), as seen in scleroderma renal crisis.
|
Laboratory Test |
Value |
Reference Range |
|
Blood urea nitrogen, mg/dL |
59.6 |
8.0 - 22.0 |
|
Complement C4, mg/dL |
11.8 |
10 – 40 |
|
Anti-Sm antibody U/mL |
2.2 |
<15 |
|
Anti-SSA antibody U/mL |
1.5 |
<15 |
|
Anti-SSB antibody U/mL |
8.5 |
<15 |
|
Antitopoisomerase I (anti- Scl-70) antibodies |
2 |
<15 |
|
Anti-Double Stranded DNA |
Negative |
|
|
IgM and IgG Anticardiolipin Antibodies |
Negative |
|
|
IgM and IgG Cytomegalovirus Antibodies |
Positive |
|
|
Cytomegalovirus PCR |
Negative |
|
|
SARS-CoV-2 RT-PCR |
Negative |
|
|
Respiratory virus antigen panel |
Negative |
|
|
Sodium, mEq/L |
135 |
|
|
Potassium, mEq/L |
4.5 |
|
|
Ionized Calcium, mmol/L |
0.79 |
1.05 - 1.3 |
|
HIV Elisa test |
Negative |
|
|
Hepatitis B Surface Antigen |
Negative |
|
|
Hepatitis C Virus antibody test |
Negative |
|
|
IgM and IgG antibodies to Trypanosoma cruzi |
Negative |
|
|
Thyroid-Stimulating Hormone, mU/L |
4.1 |
0.4 – 4 |
|
Free T4, ng/dL |
1.0 |
0.8 - 1.8 |
|
Creatine phosphokinase, U/L |
634 |
26 - 192 |
|
Total bilirubin, mg/dL |
0.76 |
< 1.2 |
|
Albumin, g/dL |
3.1 |
3.4 - 5.4 |
|
Aspartate aminotransferase, IU/L |
1939 |
5 – 40 |
|
Alanine aminotransferase, IU/L |
1806 |
19 – 25 |
The patient was treated with ACEi (Enalapril 5 mg/day) as soon as the acute renal failure allowed and continued with calcium channel blocker (amlodipine 10 mg/day), and beta-blockers (carvedilol 25 mg/12 hours). One dose of methylprednisolone (250 mg IV) was administered followed by azathioprine (50 mg/daily) stepping up to a dose of 2 mg/kg/day with a good outcome and did not require more advanced therapies, glomerular filtration rate was stable to stage 3A, LVF was also less compromise (43%) in the magnetic cardio resonance control, decrease aminotransferases (alanine: 120, aspartate: 27) and respiratory distress was markedly improved, so the patient was released from hospitalization.
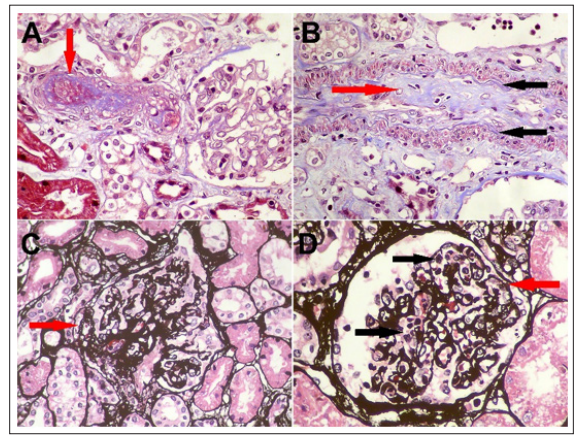
Figure 1: A) Afferent arteriole with complete obstruction of its lumen by a recent thrombus (arrow) (Masson’s trichrome stain, original magnification, X400).
In the literature SRC has been described in MCTD in 8 case reports, with a total of 9 patients, 4 (44.5%) ended in hemodialysis (HD) dependent, 1 died, and 4 responded to therapy, from these 1 just required ACEi, 1 ACEi and prostaglandin inhibitors, 1 plasma exchange, HD, mycophenolate, steroids plus ACEi, and 1 ACEi, steroids plus cyclophosphamide, presenting different results despite the use of ACEi. To the best of our knowledge, this is the tenth patient reported of scleroderma renal crisis as a complication in patients with Mixed Connective Tissue Disease. Early diagnosis is important because this evidence shows that prompt intervention is useful, leading to 60% of the patients so far known to end in a favorable outcome [4, 5].
SRC has been defined by an expert panel by systolic blood pressure >= 140 mmHg, diastolic blood pressure ≥ 90 mmHg, increase in serum creatinine by ≥ 50% over baseline, or serum creatinine ≥ 120% of the upper limit of normal for the local laboratory, proteinuria ≥ 2 + by dipstick and confirmed by spot urine protein, hematuria >2+ on dipstick or >10 red blood cells/ high power of field (in the absence of menstruation), platelet count <100,000/mm3, and hemolysis (evidenced by schistocytes or other RBC fragments on peripheral blood smear or elevated reticulocyte count), and hypertensive encephalopathy [6, 7]. LVF and myocarditis have been shown in some previous cases and the pathophysiology propose by literature is a local immune complex deposition and complement activation that drive connective tissue disorders, myocarditis, and in more advanced cases fibrosis [8]. According to the laboratory findings in the SRC an elevated plasma creatinine (96%), microangiopathic hemolytic anemia (50-60%), and thrombocytopenia are the most prevalent findings. Also has been described hyperreninemia that may explain hypertension. At the biopsy can be presences of signs of microangiopathic process and fibro intimal sclerosis with adventitial fibrosis that later may develop onion skin-like lesions, follow by hypertensive vascular damage, glomerular ischemia, thrombotic vascular occlusion, fibrosis and proliferation of intimal cells [9-11].
In our case, the patient had numerous systemic manifestations through the hospitalization, and after discarding other possible causes, the acute renal injure plus the active sediment, schistocytes on the peripheral blood smear, non-nephrotic proteinuria with the C3 hypocomplementemia lead us to the necessity to perform a renal biopsy that confirmed with the pathological findings, clinical signs, symptoms and the laboratories tests results that the patient had an SRC as a complication of MCTD newly diagnosed (high antinuclear antibodies and RNP, atherosclerosis, Raynaud phenomenon, hand edema, myopathy), we initiated treatment having a good response to the first line of therapy (ACEi), usually without any intervention, SRC will progress to end-stage renal disease in a couple of months [12].
Literature pointed out that steroids precipitate SRC, but in our case, the patient was not taking any steroids before, and with one pulse of IV steroids, ACEi and azathioprine was a notable improvement, is relevant to notice that this information of a probable correlation between steroids and SRC came from case reports with no more than 3 patients diagnosed with MCTD, and the rest were described in systemic sclerosis patients, so is require more studies that show a strong correlation between them [13-16].
In conclusion, MCTD is considered, in general, a benign illness, but when there is a lung compromise or SRC the prognosis can change, and some authors refer to that when is associated with SRC can be thought as “malignant-type MCTD” because is a life-threatening complication [17].
