Author(s): <p><span style="font-family:Georgia,serif">Jaudah Al-Maghrabi*, Haneen Al-Maghrabi, Ahmad Ghanim and Ayman Ghanim</span></p>
Sal-like protein 4 (SALL4) is a cellular pluripotent embryonic nuclear factor that has been a useful immunohistochemistry marker for germ-cell tumors.Our understanding of SALL4 expression in other human body malignancies remains limited. The diagnostic value of SALL4 expression as an independent diagnostic marker for patients with bladder urothelial carcinoma remains unclear. The aim of this study is to investigate the relation of SALL4 immunostaining in urinary bladder urothelial carcinoma and normal bladder urothelial tissue with clinicopathological diagnostic parameters.
Material and methods: Three hundred and seventy cases of bladder urothelial carcinomas and 22 non-neoplastic bladder urothelial tissues were obtained from the Department of Pathology at King Abdulaziz University Jeddah, Saudi Arabia. Tissue microarrays were constructed. Tissue sections were stained using anti-human SALL4 monoclonal antibody. The immunostaining results were recorded and analyzed.
Results: SALL4 exhibited weak nuclear expression in only 6 out of 370 cases of bladder urothelial carcinomas (1.6%). All the SALL4-positive cases were high-grade tumors. Normal urothelial tissues were all completely negative. SALL4 immunostaining revealed no association with gender, age, tumor invasiveness, stage, lymphovascular invasion, regional lymph node metastasis, or distant metastasis.
Conclusion: SALL4 is rarely expressed in bladder urothelial carcinoma and is typically limited to high-grade tumors. However, this immunoexpression must be kept in mind when applying SALL4 antibody for differentiating urothelial carcinomas from germ-cell tumors and when assessing metastatic poorly differentiated tumors of unknown origin.
Sal-like protein 4 (SALL4) is a transcription cellar factor released in stem cells during embryogenesis. It is a pluripotent zinc-finger molecule that regulates the cellular network along with OCT4 and NANOG. Normally, SALL4 interact with other cellular transcription factors such as Tbx family and SALL1 by downstreaming to manage the development of internal organogenesis of organs such as the heart, kidney, brain, limbs, and anorectal region. SALL4 is crucial for early stages of embryo development and considered a required substance for cellular proliferation in early embryonic development. SALL4 mutation with loss of functional activity is lethal for the inner cell mass. Heterozygous mutational insufficiency can result in Okihiro Syndrome, which includes renal hypoplasia, Hirschsprung’s disease, lower anogenital tract abnormalities, anencephaly, deafness, and general skeletal defects. This syndrome is closely related to acro-renal-ocular syndrome and Duane-radial ray syndrome. On the other hand, SALL4 overexpression can induce oncogenic potentials such as acute myeloid leukemia [1-4]. Although SALL4 normally remains expressed in adult germ cells of normal ovalocytes and spermatogonia, its distribution in other human cells is unknown. Detecting germ-cell tumors of the ovary, testes, mediastinum, and their metastatic sites using immunohistochemistry staining is a useful diagnostic marker for solving pathological dilemmas. However, some tumors of non-germ-cell origin have been found to express SALL4 immune staining. These tumors phenotypic expression reveals the clinical and pathological importance of studying SALL4 expression in non-germ-cell tumors. This work will further target tumor diagnoses. Some studies have suggested that SALL4 expression persists in poorly differentiated carcinomas. This nuclear positivity may mimic that of germ-cell tumors, particularly if the tumor is of unknown origin. Some studies have evaluated SALL4 expression based on cellular RNA expression rather than tissue immunohistochemistry stains. Urinary bladder urothelial carcinoma or transitional cell carcinoma (TCC) is considered to be the most common primary urinary bladder tumor. This study evaluates SALL4 expression in urothelial urinary bladder tissue with adjacent normal urothelial lining.
Paraffin wax-embedded tissue blocks of 370 primary urinary bladder urothelial carcinomas (204 high-grade and 166 low-grade) and 22 normal urinary bladder urothelial tissues were used in this study. Patients’ data and pathological materials were retrieved from the Department of Pathology at King Abdulaziz University, Jeddah, Saudi Arabia. The study was approved by the Research unit of Biomedical Ethics, Faculty of Medicine, King Abdulaziz University.
Archival paraffin-embedded bladder urothelial tissue samples and non-neoplastic urothelial tissues were collected, and selected areas were marked on Haematoxylin and eosin (H&E)-stained slides. The selected areas were representative of tumors with good cellular preservation. Areas with necrosis, crushing artifacts or poor cellular preservation were avoided. Two tissue cores with a diameter of 1.5 mm were transformed from each donor tissue block into new recipient paraffin blocks using a tissue microarray instrument (TMA Master 1.14 SP3 (3D Histech Ltd. Budapest, Hungary). Placenta tissue was used for orientation [5].
Paraffin blocks of the constructed TMAs were cut at 4μm and mounted on positive-charged Slides. Sections were deparaffinized in xylene and rehydrated in an automated immunostainer (BenchMark XT, Ventana® Medical Systems Inc., Tucson, AZ, USA). Pre-treatment was done using pre-diluted CC1 (cell conditioning solution) for 60 minutes
SALL4 (6E3) mouse monoclonal antibody (Cell Marque, 6600 Sierra College Blvd, Rocklin, CA 95677, United States) was incubated at 37°C for 20 minutes. Ventana® I-view DAB detection kit was used according to kit manufacturer instructions. A negative control reagent was used in place of the primary antibody to evaluate nonspecific staining.
Assessment of immunostaining intensity was performed semiquantitatively. The fractions of percentage of positive cells for SALL4 were divided as follows: (1) 0-25%, (2) 26-50%, (3) 50-100%. We quantified the nuclear immunostaining intensity as follows: 3 (intense brown staining), 2 (lighter staining than 3), 1 (weak staining), and 0 (no staining). A 6-point scoring system was used to categorize SALL4 expression according to a combination of intensity and extent. For the statistical analysis, an SALL4 immunostaining score of 1-3 was considered to be low (decreased) immunostaining, and an SALL4 immunostaining score of 4-6 was considered to be high (increased) immunostaining [6].
Fisher’s exact test was used to test differences between two groups of variables. The statistical analyses were performed using the SPSS® (SPSS Inc., Chicago, IL, USA) software package, version 16. A P value less than 0.05 was considered to be statistically significant
In primary urothelial tumors, a low intensity (+1) of nuclear immunostaining was detected in 6 out of 370 cases of urothelial carcinoma. They were all weak examples of nuclear staining positive for SALL4 (Figure: 1A, 1B, 1C and 1D).
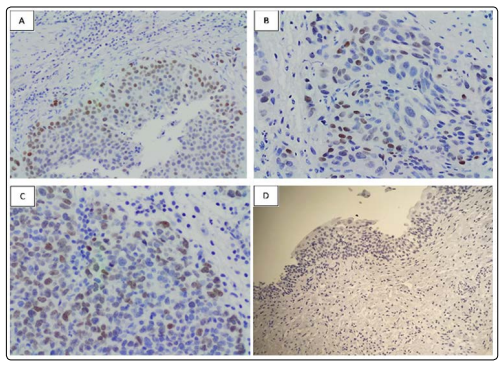
Figure 1: Immunostaining of SALL4 in urinary bladder urothelial carcinoma
A: A moderately-differentiated urothelial carcinoma showing patchy positive nuclear labelling for SALL4 (100x)
B and C: Positive nuclear staining for SALL4 in a poorlydifferentiated urothelial carcinoma (200x)
D: Negative labelling for SALL4 in normal urinary bladder urothelial lining (200x).
These positive tumors were all high-grade tumors (6/204 [2.9%]); all low-grade urothelial were negative (0/166 [0%]). Fisher’s exact test statistic value was 0.04. The result was significant at P<0.05. All normal urothelial tissues were completely negative.
Relationship between SALL4 Immunostaining and Clinicopathological Features of Urothelial Carcinoma SALL4 immunostaining revealed no statistically significant association with gender, age, tumors invasiveness, stage, lymphovascular invasion, regional lymph node metastasis, or distant metastasis.
SALL4 is a pluripotent intracellular transcription factor normally expressed in germ cells and other neoplastic human malignancies [1]. The goal of this study was to detect whether SALL4 is expressed in other non-germ-cell tumor malignancies such as urothelial carcinoma of urinary bladder. This work will make a major difference in pathologic differential diagnoses and facilitate an accurate determination of the origin of malignant tumors in daily practice. Studied SALL4 expression in normal histology tissue, germ-cell and non-germ-cell tumors. These authors performed SALL4 testing in normal human tissue. It was positively detected in germ cells, particularly testicular tissue samples, but completely absent in oral squamous epithelium, skin, thyroid, mammary, salivary glands, gastrointestinal lining, respiratory tract epithelium, liver hepatocytes, pancreas, endometrium and prostatic tissue. Revealed positive strong immunohistochemistry staining of SALL4 in germ-cell tumors such as seminomas, yolk-sac tumors and embryonal carcinomas. However, it was expressed focally in choriocarcinomas and relatively absent in more differentiated cells such as syncytiotrophoblast. However, they found that SALL4 positive cellular expression of urothelial carcinoma in 21 out of 96 cases (median 25%), all of these positive cases were highgrade urothelial carcinomas in which 20 cases were invasive carcinomas and only 1 case of in situ urothelial carcinoma. This positive cellular expression can be explained as a reflection of the oncofetal relationship of SALL4 in ureteric bud. These interesting results indicates that SALL4 positive expression is not solely indicative in germ-cell tumors or somatic cells differentiations.
Performed a study on undifferentiated tumors of uncertain origin and how to classify them by their suitable immunohistochemistry studies. They performed SALL4 immunostaining expression on 1129 Tumors, and found that SALL4 was expressed in urinary bladder urothelial carcinoma in 4 cases out of 83 cases (5%). Although SALL4 immune staining is considered recently highly sensitive and specific for germ-cell tumor diagnosis compared to others traditional germ-cell markers, caution should be taken in diagnosis of highly malignant undifferentiated tumors of uncertain origin with SALL4 positive staining. However, demonstrated that SALL4 was completely negative in metastatic urothelial carcinoma (0 expression out of 10 cases). They emphasis on the role of SALL4 cellular expression in metastatic germ cell tumors as a novel sensitive and specific marker with a particular detection utility of metastatic yolk sac tumors. Moreover, studied 3774 tumor samples, in which most of SALL4 nuclear staining were seen in testicular tumors [7-11]. Urothelial carcinoma of urinary bladder shows an interesting results; in which invasive urothelial urinary bladder carcinoma of pT2-4 showed 20% out of 60 cases SALL4 nuclear staining of weak intensity staining and 6.45% out of 62 cases in non-invasive tumors (pTa) with weak intensity nuclear staining. Our study revealed that SALL4 is expressed only in 1.6% of bladder urothelial carcinoma which less than what has been previously reported. However, the expression was limited only to high-grade tumors which is like the results of other authors. The weak nuclear staining of SALL4 expression detected in tumors of high-grade histology emphasis the role that high-grade tumors can express SALL4 nuclear staining which can make a challenge in diagnosis especially in small tissue sample lesions or core biopsy of tumors with unknown origin.
In summary, we evaluated the expression of SALL4 in urinary bladder urothelial carcinomas. We concluded that SALL4 is expressed in a restricted number of cases of urothelial carcinomas. The expression was weak and patchy and limited to high-grade tumors. However, this expression must be kept in mind when the differential diagnosis includes the classically SALL4 positive germ cell tumors while evaluating metastatic poorly differentiated tumors of unknown origin.
