Author(s): Bruno Riccardi*, Thomas De Paoli, Sergio Resta
The spread of SARS-CoV2 has been progressing continuously for over eight months. Numerous treatments have been proposed, and many have shown efficacy and ability to control the Covid-19 syndrome in this study we propose for the first time, the use of replacement therapy with Pulmonary Surfactant (PS), in patients admitted to intensive care. Pulmonary Surfactant is widely used to treat Acute Respiratory Distress Syndrome (ARDS) in protamine babies and has shown remarkable therapeutic efficacy. Since ARDS, also known as hyaline membrane disease, has many pathogenetic and symptomatological similarities with those produced by SARS-CoV2 infection, it may be useful to associate adjuvant therapy with Pulmonary Surfactant to current therapies in use. The Surfactant that we propose in our study can act as a carrier to convey even drugs with reduced absorption and poor bioavailability, directly in the most hidden alveolar areas.
SARS-CoV2 represents today one of the most important virosis of the last 10 years. The worldwide spread has created substantial health problems, mainly linked to the lack of knowledge of the characteristics of the virus, of its mode of transmission, of virulence factors, of the immune trace. We know that SARSCoV2 is transmitted by air, although it is not its only mode of transmission, and that the virus has a preferential tropism for the pulmonary district. Person-to-person spread occurs through contact with infected secretions, mainly through contact with respiratory droplets, but can also occur through contact with a surface contaminated by deposited droplets; it is not clear whether the infection can be acquired via the fecal-oral route, although there seems to be evidence to support this hypothesis. Histological examinations of the patients lungs, derived from autopsies of subjects who died of covid-19, revealed bilateral diffuse alveolar damage (DAD), pulmonary edema and hyaline membrane formation, typically indicative of acute respiratory syndrome (ARDS), as well as cells characteristic syncytial in the alveolar lumen, similar to the findings of the SARS-CoV2 outbreak of the year 2002/2003.
There is an obvious pathogenetic similarity between Acute Respiratory Distress Syndrome caused by Coronavirus and that produced for other pathogenic causes. ARDS syndrome in adults (Adult Respiratory Distress Syndrome) is generally triggered by acute pathologies that directly or indirectly damage the lung or by systemic diseases. The main causes of ARDS are: sepsis, thoracic trauma and aspiration of gastric fluids. In the initial phase, ARDS is characterized by an increase in the permeability of the pulmonary capillaries and alveolar epithelium, a phenomenon that causes an outflow of plasma and neutrophilic granulocytes within the alveolar space. This results in damage to the alveolar epithelial cells, including type II cells, responsible for the production of surfactant, with a consequent decrease in lung compliance and atelectasis. Fig.1
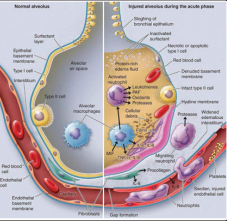
Figure1: Schematic representation of ARDS pathogenesis
However, the most common form of respiratory distress syndrome is the one that affects premature babies, called Neonatal Respiratory Distress Syndrome (NRDS), also known as hyaline membrane disease.
The events that following the SARS-CoV2 viral infection produce acute respiratory failure follow the same evolution as ARDS. SARS-CoV2 infection begins with the entry of the virus into the hosts cells. For their entry the viruses use the thorns (spikes) present on their surface and form the so-called “Corona” hence the name that characterizes this group of viruses. The spines, of a protein nature, bind to specific receptors scattered on different cell types of the host: the ACE2 receptors. From here begins the replication process of the virus and the progression of the infection. Fig. 2 to explain susceptibility to SARS-CoV2, the focus has shifted to the ACE-2 receptor (Angiotensin 2 Converting Enzyme,) the “gateway” for this Coronavirus and also for other viral agents responsible for epidemics in the past. It is an enzymatic protein. It plays a key role in the renin-angiotensin-aldosterone system (SRAA), which is the main regulator of renal excretion of salt and water, circulating plasma volume and blood pressure. ACE-2 is found on the membrane of lung cells, but also in other areas such as arteries, heart, kidney and intestines. The binding of the virus to the receptor favors its internalization in the host cell. Fig. 2
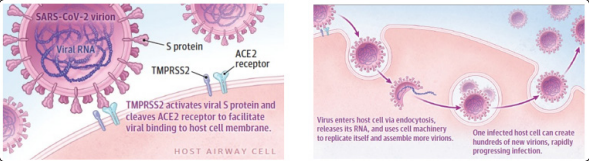
Figure 2: SARS-CoV-2 viral infection of host airway cells
From a pathogenetic point of view, SARS-CoV2 infection can cause widespread endothelial dysfunction that affects various organs and cause different pathological expressions, from acute respiratory failure to disseminated microembolism [1]. In the lungs, following the invasion of the lungs by the virus, the epithelial lining of the respiratory tree is damaged, causing inflammation. As the inflammation progresses, damage to the epithelium of the respiratory tract occurs, reaching the capillaries that line the pulmonary alveolus, the terminal portion of the respiratory bronchioles. Once infected and inflamed, the capillaries exude inflammatory material into the pulmonary alveoli [2,4]. The lungs, which progressively fill with inflammatory material, are unable to receive enough oxygen for the local and systemic circulation. This phenomenon generates a clear reduction in peripheral blood O2 saturation with difficulty in disposing of carbon dioxide. This cascade of pathophysiological events is the leading cause of death following severe SARS-CoV2 pneumonia. In 15-20% of cases, the immune systems response to lung inflammation can cause what is known as a “cytokine storm”. This fleeting syndrome can cause more damage to cells in the body than to the virus it is trying to defeat, and is believed to be the main reason why patients conditions, even in healthy individuals, can rapidly deteriorate. Fig. 3 [5-9]. if the alveoli collapse, the patient should undergo assisted ventilation.
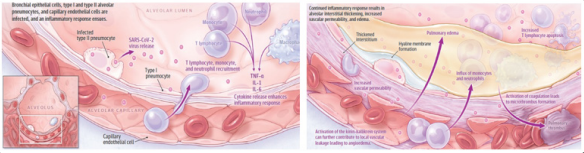
Figure 3: Current understanding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)
The main pathological features in lungs included extensive impairment of type I alveolar epithelial cells and atypical hyperplasia of type II alveolar cells, with formation of hyaline membrane, focal hemorrhage, exudation and pulmonary edema, and pulmonary consolidation. The mucous plug with fibrinous exudate in the alveoli and the dysfunction of alveolar macrophages are characteristic abnormalities. Fig. 4
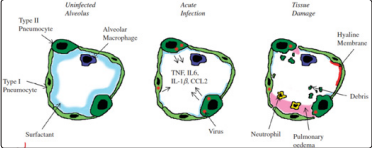
Figure 4: Model of an infected alveolus in the lung
The most common symptoms produced by the SARS-CoV2 viral infection are: hyperthermia, dry cough, fatigue, mucosal expectoration, dyspnoea, sore throat (14%), headache (14%), muscle pain (14%), chills (11%). Less frequent are: nausea and vomiting (5%), stuffy nose (5%) and diarrhea (4%). In the most serious cases, almost all found in subjects already suffering from previous pathologies, interstitial pneumonia develops, acute renal failure, up to death. As ARDS progresses to the acute phase, alveolar flooding (edema), interstitial inflammation and compression atelectasis are observed, as well as an increase in the fibrous volume of the lung tissue and a decrease in the volume of lung gases [3]. COVID-19 patients suffering from ARDS / ALI (Acute Lung Injury) often require intubation and mechanical ventilation to offset respiratory distress as increasing hypoxemic respiratory insufficiency generates widespread acute alveolar damage [10-11]. The initiation and development of ARDS / ALI depends on the activation of the inflammasomes. Inflammasomes are an integral part of our innate immune system. Inflammasomes detect pathogens and their activation releases pro-inflammatory cytokines: interleukin (IL) 1β and IL 18. Recently, the NLRP3 inflammasomes has been identified as a key to the induction of ADRS / ALI. Interleukin 1 beta (IL-1β) is a potent proinflammatory cytokine implicated in the pathogenesis of acute respiratory distress syndrome. The production of IL-1β is strictly controlled and depends on the activation of the NLRP3 inflammasomes. All viruses code for proteins that can interfere with the innate immune system. Interference can inhibit or enhance host immune responses. Some viruses disrupt the immune system to promote evasion and pathogenicity, while others modulate cellular factors that also disrupt immune responses. Viroporins E of SARS-CoV viruses are proteins that form protein-lipid channels in cell membranes such as to allow the passage of calcium ions. These ion channel movements involving calcium are specific triggers in the activation of NLRP3 inflammasomes, resulting in overproduction of proinflammatory IL-1β cytokines. The transport of calcium through these protein E ion channels initiates the cytokine production cascade capable of generating uncontrollable cytochemical storms and ARDS / ALI resulting in bilateral interstitial pneumonia. Ionic disorders at the cellular level are the reason why coronaviruses such as SARS-CoV2 can have a destructive impact in causing severe immunopathological consequences that in the early stages of the virus were out of control in infected patients.
Surfactant is a fluid produced by the alveolar epithelium of the lungs and plays an essential role in respiratory dynamics. It covers, like a thin film, the inner wall of the alveoli and plays the fundamental role of reducing the surface tension at the air / liquid interface, thus preventing alveolar collapse in the exhalation phase of the respiratory cycle. Surfactant deficiency is the major cause of respiratory distress syndrome (RDS) in infants. Chronic lung disease can be caused by numerous changes that damage lung tissue and are often associated with inflammatory processes that alter the normal function of pulmonary surfactant (PS). PS is a lipid-protein material that covers the entire respiratory surface of mammals. Fig. 4
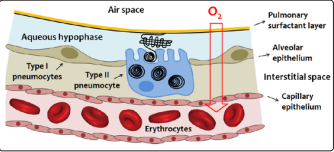
Figure 4: Schematic representation of the respiratory blood-gas barrier
The surfactant, as already described above, is a mixture of phospholipids and structural proteins, it is synthesized by type II pneumocytes and accumulated in the lamellar bodies, intracellular organelles in which secretion then takes place, with the fusion of their outer membrane with the apical plasma membrane of the cell, and with consequent diffusion of the surfactant in the alveolar space. Surfactant (SP) Proteins: In recent years, SPs have been the subject of particularly in-depth studies. There are 4 proteins associated with surfactants: SP-A, SP-B, SP-C and SP-D: two of these (SP-A, SP-D) are water-soluble, the other two are hydrophobic. Fig. 5
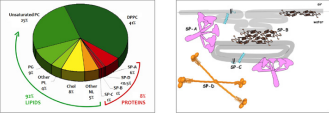
Figure 5: Typical pulmonary surfactant composition, Structural models of surfactant proteins
SP-A is a lectin made up of 18 monomeric molecules grouped together in a sort of bouquet of flowers. (Fig. 5 right) Some of the recognized functions of SP-A intervene in the metabolic cycle of surfactant, participating in the process of converting the lamellar bodies into tubular myelin within the air spaces. SP-A also appears to be involved in regulating the flow of surfactant into and out of type II cells by binding to a specific receptor present on these cells. It also plays an important role in improving the resistance to surfactant inhibition and in stimulating alveolar macrophages, therefore it seems to play a fundamental action in the defense mechanisms of the lung. In particular, two proteins play a critical role for the surfactant properties of the surfactant, the surfactant proteins B (SP-B), and C (SP-C), which represent about 4% of the total and are encoded respectively by the SFTPB and SFTPC. Mutations in the genes encoding these proteins are the cause of acute neonatal respiratory failure and pulmonary interstitial disease in the infant and child.
Although the composition of PS varies between different species and environmental conditions, it consists mainly of phospholipids (80-90%), mostly saturated dipalmitoyl-phosphatidylcholine (DPPC), neutral lipids (6-10%) among which the cholesterol (Cho) is the most abundant molecule; and at least four specific proteins (5-10%) of which two are hydrophilic (SP-A and SPD). The main function of the surfactant is to allow the rhythmic expansion of the alveoli during breathing and to prevent their collapse during exhalation, however the surfactant also participates in the response of the alveoli to a large variety of external attacks such as inflammation, infections and oxidative stress. In the lungs there is an interface between the liquid that covers the alveolar walls and the gas inside them. Also in this case, the surface liquid layer tends to contract and expel the air to the outside with consequent collapse of the alveoli. The pressure generated inside these due to the surface tension is called collapse pressure, and can be estimated through Laplaces law.
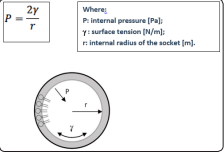
Figure 6: Diagram of the forces acting on the internal surface of the alveolus. Lo is represented in gray aqueous layer that covers the alveolar wall; the surfactant molecules are partially immersed in it, as illustrated on the left. P: pressure, ɣ: surface tension, r: internal radius of the alveolus
The collapse pressure is, according to Laplaces law directly
proportional to the surface tension: the more the latter increases,
the greater the pressure to overcome to avoid the collapse of the
alveoli and is inversely proportional to the radius of the alveolus.
In the physiological lung, the surface tension is greatly reduced
by the presence of the surfactant. The absence of surfactant can
lead to atelectasis (collapse of the alveoli).
To summarize it can be said that the surfactant performs important
functions by reducing the surface tension:
1. increases lung compliance and therefore reduces the work
required for lung expansion;
2. Contributes to the stability of the alveoli by preventing their
collapse;
3. Limits the risk of pulmonary edema by decreasing the recall
force of liquids within the alveolus;
In analogy with liposomes, i.e. nanometric vesicles composed of a phospholipid bilayer that encloses an aqueous core, the incorporation of different classes of drugs into proteolipid vesicles consisting of PS components has been considered a promising strategy for years. Following the concept of using liposomes as a way to encapsulate and enhance the bioavailability of hydrophobic compounds, it has been proposed that liposomes Surfactants facilitate the release of poorly water-soluble drugs into the lung air spaces [12]. However, the relative efficacy of these lipid / surfactant drug combinations has been poorly evaluated. Recent studies have shown that the intrinsic ability of lipid-PS protein complexes to adsorb and diffuse along air-liquid interfaces can certainly be used to facilitate local interface-assisted drug delivery. Surfactant deficiency is the major contributor to infant respiratory distress syndrome (RDS) and adult respiratory distress syndrome (ARDS). Since 1980, the exogenous administration of surfactant for the treatment of these syndromes in children is being studied.
After a devastating impact, secondary to the lack of knowledge of the viral variant caused by the Covid-19 syndrome, numerous treatments have been used on an experimental basis, up to the formulation of effective pharmacological schemes able to intervene in every phase of the disease. We mention a few [37-38]:
From the foregoing, the effects of ARDS from SARS-CoV-2 or other pathologies produce a common devastating effect on the respiratory level: the destruction of the alveolar epithelium with the collapse of the respiratory cavity and related acute respiratory failure.
PROSURF extractive surfactant produced and patented by the Argentina Company LIPOTECH S.A./ NIALTEC SA, (www. lipotech.com.ar), already used in the treatment of pulmonary distress syndrome in premature babies.
PROSURF is marketed in Latin America as a drug under the
following brand names:
In Argentina
1. Baby Fact B: GeMePe SA
2. Natsurf: Laboratorios Richet SA.
3. Surfactante B Richet: Laboratorios Richet SA.
In Brazil
1. Baby Fact P: GeMePe SA
2. Surfactante P Richet: Laboratorios Richet SA.
PROSURF has the following composition:
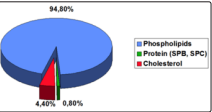
Figure 7: Composition of Prosurf
PROSURF Pulmonary Surfactant is a natural complex extracted
from bovine lungs, washed and purified and is composed of
phospholipids, neutral lipids and four specific proteins (SP-A,
SP-B, SP-C and SP-D). Fig. 7 Surfactant plays a vital role in
lung physiology with two types of functions previously outlined
Biophysical: Avoid alveolar collapse and bronchial obstruction
during normal or breathing forced.
Immunological: Protects the lungs from injuries and infections
caused by the inhalation of particles and microorganisms.
Administration of exogenous surfactant has become standard
therapy for infants with respiratory distress syndrome (RDS)
or jaline membrane disease PROSURF has demonstrated in
numerous clinical studies, using the appropriately marked product,
a high distribution capacity at the alveolar level and resolution of
respiratory failure. [17-36], Fig. 8
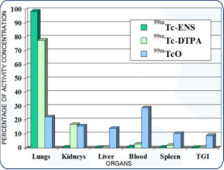
Figure 8: Distribution of PROSURF in various organs
C Solana, et al reports that treatment with this surfactant has rapid effects that improve the outcome of memnbrane jaline disease. No adverse effects could be found related to the administration of surfactants.
Eight months after the start of the SARS-CoV-2 pandemic, we propose the use of the replacement Pulmonary Surfactant, as an adjuvant to the therapies currently in use to contain the symptoms and prevent the most damaging effects of ARDS, until they are available. Truly effective drug therapies or vaccines. PROSURF can represent a valid support, both for the demonstrated efficacy in overcoming the critical effects of acute respiratory syndrome, and for the ease of administration by inhalation. It can also be used as a carrier to convey drugs with reduced absorption, which will thus be able to reach the entire alveolar area with maximum bioavailability.
The authors report no conflicts of interest in this work.
1. Huertas A, Montani D, Savale L, et al. (2020) Endothelial
cell dysfunction: a major player in SARS-CoV-2 infection
(COVID-19)?. Eur Respir J.
2. Chaofu Wang, et al. (2020) Alveolar macrophage dysfunction
and cytokine storm in the pathogenesis of two severe
COVID-19 patients, E Bio Medicine 57: 102833.
3. Alain C Borczuk, et al. (2020) COVID-19 pulmonary
pathology: a multi-institutional autopsy cohort from Italy
and New York City, Modern Pathology.
4. Lisa E Gralinski, Ralph S Baric (2015) Molecular pathology
of emerging coronavirus infections , J Pathol 235: 185-195.
5. Robert J. Mason (2020) Pathogenesis of COVID-19 from a
cell biologic perspective, Eur Respir J in press.
6. Robert J Mason (2020) Thoughts on the alveolar phase of
COVID-19, Am J Physiol Lung Cell Mol Physiol 319: L115-
L120.
7. Giacomo Grasselli, et al. (2020) Pathophysiology of
COVID-19-associated acute respiratory distress syndrome:
a multicentre prospective observational study, thelancet.com/
respiratory Published online.
8. Xiaobo Yang, et al. (2020) Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan,
China: a single-centered, retrospective, observational study,
Lancet Respir Med, 2020 Published Online.
9. To S Fung, Ding X Liu (2014) Coronavirus infection,
ER stress, apoptosis and innate immunity, Frontiers in
Microbiology Virology 5: 296.
10. Firas A Rabi, et al. (2020) SARS-CoV-2 and Coronavirus
Disease 2019: What We Know So Far, Pathogens 9: 231.
11. Ritwick Mondal, et al. (2020) COVID-19: Are we dealing with
a multisystem vasculopathy in disguise of a viral infection?.
Journal of Thrombosis and Thrombolysis.
12. Roberta Guagliardo, et al. (2018) pulmonary surfactant and
drug delivery: Focusing on the role of surfactant Proteins,
Journal of Controlled Release 291: 116-126.
13. Alejandra Cimato et al. Analysis of the structure and surfactant
activity of novel formulations containing exogenous
pulmonary surfactant and glucocorticoids, Respiratory
Physiology & Neurobiology 233: 33-40.
14. Yi Y. Zuo, et al. (2008) Current perspectives inpulmonary
surfactant-Inhibition, enhancement and evaluation,
Biochimica et Biophysica Acta 1778: 1947-1977.
15. Meeting (2002) LUSO DEL SURFATTANTE NATURALE
NELLA PATOLOGIA RESPIRATORIA ACUTA Cagliari, 27
maggio 2002, Interactive Medicine , Supplemento- Settembre
2002.
16. Elena Lopez-Rodriguez, Jesus Pérez-Gil (2014) Structurefunction relationships in pulmonary surfactant membranes:
From biophysics to therapy, Biochimica et Biophysica Acta
1838: 1568-1585.
17. J L do Campo, E G Bertranou, A De Lorenzi, A A Hager
(1994) Nebulised exogenous natural surfactant after cardic
surgery. The Lancet 343: 482.
18. JL do Campo, et al. (1994) Natural surfactant aerosolisation
in adult respiratory distress syndrome. The Lancet. 344: 413-
414.
19. Alak O Degrossi, et al. (1996) (199) Use of lung surfactant-
99mTc in lung studies. Nuclear Medicine. Clinic Aspect
233-236.
20. G Calmanovici,et al. (1998) 99mTc-ENS, a New
Radiopharmaceutical for Aerial Lung Scintigraphy:
Comparative Studies In Rats. Nuclear Medicine & Biology
25: 511-513.
21. G Calmanovici, et al. (1998) El sistema surfactante
pulmonar: Fisiología, patologías asociadas a su alteración
y administración exógena como agente terapéutico y de
diagnóstico. Acta Phisiol. Pharmacol. Ther. Latinoamericana
(APPTLA) 48: 175-190.
22. G Calmanovici, et al. (1998) 99mTc-ENS: A New
Radiopharmaceutical for aerial Lung Scintigraphy. Eur J
Nuclear Medicine. 25: 891.
23. G Calmanovici, et al. (1999) 99mTc-ENS: A New
Radiopharmaceutical for Aerosol Lung Scintigraphy.
Comparison between Different Freezes Dried Formulations.
The Journal of Nuclear Medicine. 6: 1080-1083.
24. G Calmanovici, et al. (2000) 99mTc-ENS Ventilation scintigraphy. Preliminary study in human volunteers. Nuclear
Medicine & Biology. 27: 215-218.
25. G Calmanovici, et al (2001) Centellografía aérea pulmonar.
Estudio comparativo con 99mTc-ENS y 99mTc-DTPA
Diagnóstico 51-54.
26. G Calmanovici, et al (2001) La medicina nuclear en el
diagnóstico de las enfermedades pulmonares. Revista
Farmacéutica 143. 7-14.
27. G Calmanovici, et al. (2002) Formas farmacéuticas del
surfactante natural exógeno (ENS) para ser utilizado en
diagnóstico y terapia. Revista Farmacéutica. 144: 11-14.
28. G Calmanovici, et al. (2003) Evaluation of Different
Formulations to Lebel Exogenous Natural Surfactant Labeled
With (99mTc-ENS). Stability and Toxicity Studies, Alasbimn
Journal. 2003; Year: 5, N°: 19.
29. G Calmanovici , et al. (2004) Quality control methods for
99mTc-labeled exogenous natural surfactant (99mTc-ENS).
Journal Label. Compd. Radiopharm. 47: 47-53.
30. Mayosky, G. Saravia, et al. () 99mTc-ENS vs. 99mTc-DTPA
as Aerosol Lung Scintiscanning Agents. Alasbimn Journal.
April 2004, Year: 6 N: 24.
31. Calmanovic, et al. (2005) Quality Control Validation for
Exogenous Natural Surfactant Labeled witj 99mTc. Journal
of Nuclear Medicine Technology. 33: 234-237.
32. María Martínez Sarrasague, et al. (2011) Influence of serum
protein and albumin addition on the structure and activity of
an exogenous pulmonary surfactant. Respiratory Physiology
& Neurology. 175 316-321.
33. María Martínez Sarrasague, et al. (2012) Effect of serum
proteins on an exogenous pulmonary surfactant: ESR analysis
of structural changes and their relation with surfactant activity.
Respiratory Physiology & Neurology. 183: 48-57.
34. María Martínez Sarrasague, et al. (2013) Effect of serum
lipoproteins and cholesterol on an exogenous pulmonary
surfactant. ESR analysis of structural changes and their
relation with surfactant activity. Respiratory Physiology &
Neurology 189: 581-587.
35. Alejandra Cimato, et al (2016) Analysis of the structure
and surfactant activity of novel formulations containing
exogenous pulmonary surfactant and glucocorticoids.
Respiratory Physiology & Neurology 233: 33-40.
36. Alejandra Cimato, et al. (2018) Developing an exogenous
pulmonary surfactant-glucocorticoids association: Effect of
corticoid concentration on the biophysical properties of the
surfactant. Respiratory Physiology & Neurology 247: 80-86.
37. Tim Smith, et al. (2020) COVID-19 Drug Therapy - Potential
Options, Clinical Drug Information.
38. Gao J, T Zhenxue, Yang X (2020) Breakthrough: Chloroquine
phosphate has shown apparent efficacy in treatment of
COVID-19 associated pneumonia in clinical studies. Biosci
Trends 14: 72-73.scintigraphy. Preliminary study in human volunteers. Nuclear
Medicine & Biology. 27: 215-218.
