Author(s): Azmat Karim*, Mohammad Shameem, Anjana Talwar, Deepak Talwar and Abdul Allam Khan
Aim: To evaluate the effect of Mepolizumab on COVID-19 patients with severe eosinophilic asthma and other comorbidities.
Methods: 11 patients on Mepolizumab to treat SEA and contracted COVID-19 were taken. The retrospective clinical, demographical, and biochemical data were collected and evaluated.
Results: Out of the 11 patients infected with COVID-19, 6 were males and 5 were females, with a mean age of 58. The most common risk factor was Hypertension (54%), Diabetes (36%), Obstructive sleep apnea (36%), and coronary artery disease (27%). Four out of 11 are above the age of 60, and half of the patients were overweight. Despite having multiple comorbidities, none of the patients required admission to the hospital.
Conclusions: Patients who were administered mepolizumab and were diagnosed with COVID-19 only experienced mild symptoms and did not suffer from any complications despite having multiple comorbidities. This suggests that there may be a positive effect of mepolizumab on the course of COVID-19 or that it could be associated with asthma. However, further, larger-scale studies are required to determine the role of mepolizumab in COVID-19 and to explore its potential as a protective effect against the disease
Since the outbreak of COVID-19, it has been responsible for millions of deaths and continues to cause mortality and morbidity throughout the globe. The presence of comorbidities such as diabetes mellitus, cardiovascular disease, or hypertension is associated with more severe complications and a higher case fatality rate in COVID-19 [1-3]. However, asthma has not been identified as an important risk factor for COVID-19 [3-4].
COVID-19 patients with eosinophilia have a lower incidence of complications and is associated with a better prognosis [5]. Thus, the peripheral blood eosinophil count could be considered a possible biomarker for evaluation and prognosis [6]. The role of biologics has been established in Severe Eosinophilic Asthma(SEA). Still, concern has been expressed as eosinopenia due to treatment may be a risk factor for worse disease outcomes [7,8].
Different reports noted that treatment with benralizumab, dupilumab, and omalizumab is not associated with a significant negative impact during active COVID-19 infection in severe asthma [9-11]. The expert recommendation is to continue biologic therapy unchanged in SEA [12,13]. The data on mepolizumab and its effect on COVID-19 is very limited; hence, we present a case series of 11 SEA patients on mepolizumab who encountered COVID-19 and their outcomes.
The emergence of the Delta variant of COVID-19 was detected, resulting in a declaration of public health emergency due to its rapid transmission. In order to combat the virus, hospitals implemented standard operating procedures, as recommended by the Indian Council of Medical Research (ICMR) and the World
Health Organization (WHO), for emergency measures against all COVID-19 variants [14].
The study’s primary goal is to evaluate the effect of Mepolizumab on COVID-19 patients with SEA, although some patients co- exist with other comorbidities. A total of 11 patients who were on mepolizumab to treat SEA and contacted COVID-19 were taken. Their clinical, demographical, and biochemical data were collected retrospectively.
A total of 11 patients who were being treated with mepolizumab for SEA and diagnosed with COVID-19 caused by the Delta strain were included in the study conducted between April 2020 and April 2021. contracted COVID-19 were included in the study. The group consisted of 6 males and 5 females. The patients were managed according to the guidelines issued by the ICMR for individuals with comorbidities and those at high risk of developing severe COVID-19 [15].
The study included 6 males and 5 females, with mean age of (58.27±12.44). Four out of 11 were above the age of 60, and half of the patients were overweight. Hypertension was the most common risk factor (54.54%), followed by Type 2 diabetes (36.36%), Obstructive sleep apnea (36.36%), and coronary artery disease (27.27%) (as shown in Table 1).
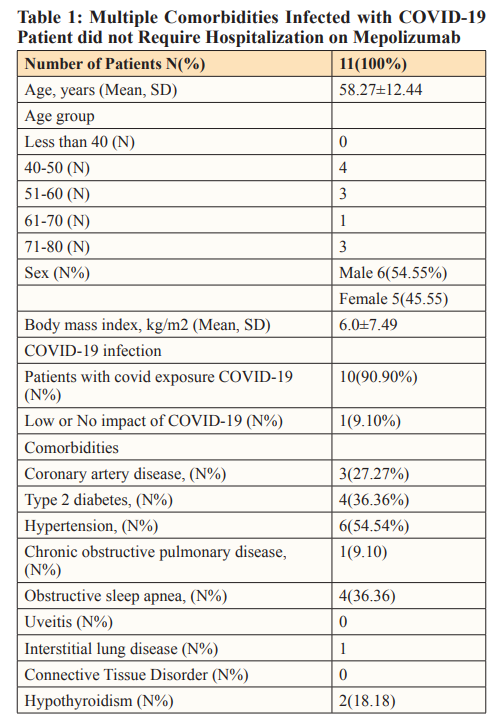
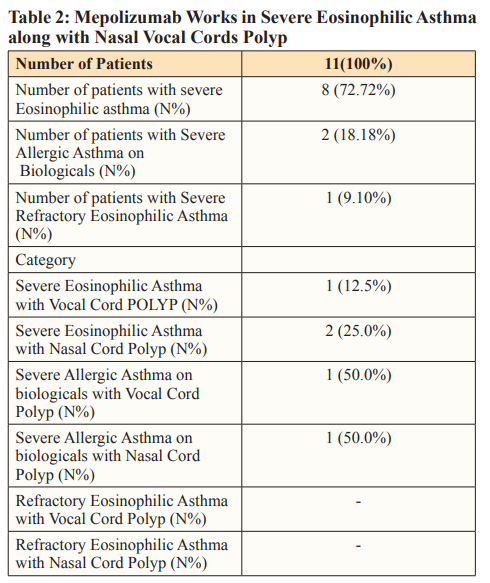
All the patients were infected with COVID-19, SEA, and even with multiple comorbidities (Table 2); they did not require admission to the hospital.
In our paper, we present a series of patients who had COVID-19 while receiving mepolizumab for treatment of SEA. Our patients had various risk factors, such as Type 2 diabetes, coronary artery disease, hypertension, and obstructive sleep apnea, which are associated with worse clinical outcomes in COVID-19. In accordance with WHO and ICMR guidelines, patients over the age of 58 years are susceptible to severe infection and mortality and are advisable for hospitalization. Further, all the patients in the study group also co-exist with different comorbidities. Thus, the patients needed continuous monitoring and specialized medical care. The fact that none of our patients required admission due to COVID-19 despite multiple comorbidities suggests asthma or the drugs used may have had a protective role in the course of COVID.
A study conducted in Chicago showed that the risk of death in asthmatic patients is similar to that of non-asthmatic individuals who have no other risk factors [16]. Patients who have Th2-asthma phenotype, which is characterized by high levels of peripheral eosinophils (more than 150 cells/ml), are less likely to be admitted to the hospital and also have a lower mortality rate [17].
The use of biologics in patients of SEA has been well established, but their effects on COVID-19 patients are limited. Biologics can modulate, decrease, or deplete circulating eosinophils and thus should be detrimental to COVID-19 patients. Still, their use has been associated with positive outcomes and decreased hospital admissions. In a review article of around 98 patients of SEA on biologics, only one mortality was seen, attributed to patient age and multiple comorbidities [18]. Some cases reported a milder course of COVID-19 in patients with severe asthma on biologic drugs, indicating the possibility of some unknown mechanisms of asthma or drugs used in asthma that positively affect the course of COVID-19 [9-11,19]. A case series’s fundamental goal must be to develop hypotheses that can be evaluated in studies with more methodological precision. A case series is a screening tool for probable hypotheses needing further investigation. Case series are designed to be descriptive; descriptive statistics can be employed primarily. Hence, various confounding factors are not considered for comparative statistical analysis and interpretation [20].
Our study concluded that patients undergoing treatment with mepolizumab and contracted COVID-19 had a mild form of the disease and did not experience any complications, even though they had multiple comorbidities and severe chronic diseases at the time of the infection. The patients did not develop a secondary infection, and it is possible that treatment with mepolizumab could have played a role in preventing the severity or mortality of COVID-19. This could be associated with the positive effect of mepolizumab on COVID-19 or its potential use in treating asthma. However, larger prospective studies are needed to determine the precise role of Mepolizumab in COVID-19 and to explore the potential protective effects of the disease.
Nil
1. Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239-1242.
2. Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A (2020) Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med 8: 436-438.
3. Li X, Xu S, Yu M, Wang K, Tao Y, et al. (2020) Risk factors for severity and mortality in adult COVID-19 in patients in Wuhan. J Allergy Clin Immunol 146: 110-118.
4. Caminati M, Lombardi C, Micheletto C, Elena Roca, Barbara Bigni, et al. (2020) Asthmatic patients in COVID-19 outbreak: few cases despite many cases. J Allergy Clin Immunol 46: 541-542.
5. Gonzalez MM, Gonzalo ES, Lopez IC, Fernández FA, Pérez JLB, et al. (2021) The prognostic value of eosinophil recovery in COVID-19: a multicentre, retrospective cohort study on patients hospitalised in Spanish hospitals. J Clin Med 10: 305.
6. Roca E, Ventura L, Zattra CM, Lombardi C (2021) Eosinopenia: an early, effective and relevant COVID-19 biomarker? QJM 1140: 68-69.
7. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, et al. (2020) Clinical characteristics of 140 patients infected with SARS- CoV-2 in Wuhan, China. Allergy 75: 1730-1741.
8. Du Y, Tu L, Zhu P, Mu M, Wang R, et al. (2020) Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 201: 1372-1379.
9. Lommatzsch M, Stoll P, Virchow JC (2020) COVID-19 in a patient with severe asthma treated with omalizumab. Allergy 75: 2705-2708.
10. García Moguel I, Díaz Campos R, Alonso Charterina S, Fernández Rodríguez C, Fernández Crespo J (2020) COVID-19, severe asthma, and biologics. Ann Allergy Asthma Immunol 125: 357-359.
11. Bhalla A, Mukherjee M, Radford K, Nazy I, Kjarsgaard M, et al. (2021) Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology. Allergy 76: 957-958.
12. Bousquet J, Jutel M, Akdis CA, Klimek L, Pfaar O, et al. (2021) ARIA-EAACI statement on asthma and COVID-19 (June2-2020). Allergy 76: 689-697.
13. Klimek L, Pfaar O, Worm M, Eiwegger T, Hagemann J, et al. (2020) Use of Biologicals in allergic and type-2 inflammatory diseases during the current COVID-19 pandemic: position paper of Ärzteverband Deutscher Allergologen (AeDA) A, Deutsche Gesellschaft für Allergologie und Klinische Immunologie (DGAKI)B, Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA)C, Österreichische Gesellschaft für Allergologie und Immunologie (ÖGAI) D, Luxemburgische Gesellschaft für Allergologie und Immunologie (LGAI)E , Österreichische Gesellschaft für Pneumologie (ÖGP)F in co-operation with the German, Austrian, and Swiss ARIA groupsG, and the European Academy of Allergy and Clinical Immunology (EAACI)H. Allergol Select 4: 53-68.
14. Rajendran S, Panneerselvam RK, Kumar PJ, Rajasekaran VA, Suganya P, et al. (2022) Prescreening and triage of COVID-19 patients through chest X-ray images using deep learning model. Big Data 3: 1-12.
15. National guidelines for ethics committees reviewing biomedical&health research during COVID-19 pandemic. Indian Council of Medical Research, April 2020. https://main.icmr.nic.in/sites/default/files/guidelines/EC_Guidance_COVID19_06_05_2020.pdf
16. Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, et al. (2020) Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol 146: 307-314.
17. Ferastraoaru D, Hudes G, Jerschow E, Jairwala S, Karagic M, et al. (2021) Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract 9: 1152-1162.
18. Lombardi C, Bagnasco D, Passalacqua G (2020) COVID-19, Eosinophils, and Biologicals for Severe Asthma. Front Allergy 28: 859376.
19. Forster Ruhrmann U, Szczepek AJ, Bachert C, Olze H (2020) COVID-19 in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J Allergy Clin Immunol 146: 218-220.
20. Kooistra B, Dijkman B, Einhorn TA, Bhandari M (2009) How to design a good case series. J Bone Joint Surg Am 91: 21-26.
View PDF