Author(s): <p>Afnan Sayeid Himi, Hasan Mahmud, Fokhrul Islam, Mohiuddin Ahmed Bhuiyan, Mohd Ashraful Islam and FatemaTuz Zohora*</p>
An ophthalmic drug delivery system is a method used to treat various ocular diseases and vision related conditions. It is used to treat macular degeneration, viral infections, glaucoma, ocular inflammations, dry eye syndrome and retinal degenerations. In this drug delivery system face to both the pharmaceutical and medical sciences significant obstacles. The primary challenge is maintaining an effective medicine concentration at the therapeutic target for extended periods. The corneas anatomy, physiology and barrier function work together to prevent rapid drug absorption. The various barrier properties overcoming the eye defenses without causing permanent tissue damage. Topical, systemic, periocular and intravitreal barriers are the main ophthalmic administration route. Some approaches are adding binding agents by molecular imprinting and entrapment of vesicular systems into the lens matrix. Various elimination mechanisms make it difficult for conventional ophthalmic drug delivery to the retinal tissues. Current ophthalmic drug delivery systems with lower bioavailability and invasive nature, pose challenges for novel technologies to improve treatment for ocular disorders. Hydrogels, polymer micelles, nanoparticles (NPs), implants, microneedles, ion electrophoresis, ultrasound and more new ophthalmic drug delivery methods have recently been developed. These systems are claimed to offer extended ocular surface residence time, thereby reducing the impact of natural eye clearance systems. Modern methods increase the therapeutic effectiveness of the ophthalmic route. Advances in nanotechnology and biomaterials science may development of smart technologies for enhancing ophthalmic drug delivery. This system would likely be more pseudoplastic than the materials currently in use and more hydrophilic to reduce blink noise.
The ophthalmic drug delivery system is a crucial administration route [1]. The pharmaceutical and medical sciences face significant obstacles in the ophthalmic drug delivery [2]. The eye is extremely resistant to external chemicals due to its structural and functional features. Formulators face difficulties in obtaining the eyes defenses without resulting in long-term tissue damage [3]. The primary challenge is maintaining an effective medicine concentration at the therapeutic target for extended periods [4]. An ideal delivery scheme should be improved drug bioavailability and controlled release at the site of action. It overcomes various ocular barriers [5]. Topical, systemic, periocular and intravitreal barriers are the main ophthalmic administration route [4]. The barrier properties overcoming the eye defenses without causing permanent tissue damage [3]. The most popular technique for treating ocular diseases is topical route of administration [6]. Eye disorder patients’ health and quality of life have been put at risk. The main cause of handicap in the world today is vision loss brought on by ocular illnesses [7]. The primary issue is the rapid pre-corneal loss. Pre-Corneal loss is resulting from nasolacrimal drainage and high tear fluid turnover. The drug concentrations were only 10% present [8]. The scaling up of nano emulsions is accompanied by the stability issue, high cost and time-consuming preparation procedures [9]. The cause of this is the quick drug clearance caused by blinking and lachrymation. Blinking and lachrymation are accelerated by administering non-physiological quantities of drugs to the eye. Additionally, it has been discovered by various variables. These variables are blamed for the medications limited bioavailability following topical ophthalmic drug delivery [10]. The major cause of irreversible blindness is retinal disease [11]. A few examples are age-related macular degeneration (AMD), proliferative vitreoretinopathy (PVR), diabetic macular edema (DME), endophthalmitis, cytomegalovirus (CMV), retinitis and retinitis pigmentosa [12]. Various elimination mechanisms make it difficult for conventional ophthalmic formulations to adequately deliver drugs to the retinal tissues [13]. Eye drops address complicated penetration barriers corneal, nasolacrimal drainage, protein binding, systemic absorption, enzymatic degradation blood-aqueous (BAB) and blood-retinal (BRB) [14]. Following topical administration, these have an average 5% low ocular bioavailability [15]. Some approaches are adding binding agents by molecular imprinting and entrapment of vesicular systems into the lens matrix [16]. Nanotechnology is a crucial and promising method for ophthalmic drug delivery. The anterior and posterior segment is now being done with this technique [1]. The ophthalmic control drug delivery systems are gels, ointments, liposomes, microspheres, ocular mini tablets (MT) or films, micro- and nanoparticles [17]. The formulation utilized most frequently for ophthalmic medication administration is topical eye drops. For the ophthalmic method, antibiotics are frequently used as a solution or as an ointment [2]. Topical, sub tenon, intraocular or subconjunctival administration of antibiotics can be used to treat ocular infections [18]. The impact of antibiotic administration on bacterial resistance to antimicrobial stress can be observed [2]. Several new ophthalmic drug delivery methods have recently been developed. These include hydrogels, polymer micelles, nanoparticles (NPs), implants, microneedles, ion electrophoresis, ultrasound and more [4]. Numerous experiments were conducted to create effective ophthalmic drug delivery systems (ODDS). ODDS enhances the duration of treatment, targeting and adherence [19]. The World Health Organization estimates that one person worldwide loses their vision every five seconds. Globally, 1.3 billion people suffer from vision problems [20]. Over the past few decades, the dosage formulations used today have improved. Ocular dosage forms must be sterile, secure and free of the patient’s allergies [2].
In this review, I have summarized mainly the most recent challenges, approaches and application factors in various types of ophthalmic drug delivery system. I have also gotten knowledge about recent and current ophthalmic drug delivery system. Finally, I provide input for future ophthalmic drug developments to treat ocular diseases.
Solutions are the most frequently used. The substance must be effective on the surface or passing through the cornea or conjunctiva. The drug in solution is dissolved state. It can take effect immediately [21].
Gel in situ was creating novel ideas of production. It was produced in the early 1980’s. It is increasing a drug formulation’s viscosity in the precorneal region [21].
Mydriatics or cycloplegics can be used alone or in combination as an eye spray is not a common practice. For cycloplegic examinations or to dilate the pupil, these sprays are used in the eyes [21].
Ocular inserts are a solid dosage form. This overcomes the drawback of conventional ophthalmic systems. It enables more effective, continuous and controlled drug delivery [21].
Contact lenses can absorb water-soluble drugs. The substances are saturated to release the drug slowly over a long period of time [22]. Hydrophilic contact lenses can lengthen the drugs ocular residence duration [21].
Collagen shields were first created as a bandage for the cornea and are made from the skin of a calf during pregnancy. The implants dissolve within 10, 24, or 72 hours. It has been softened by the tear fluid and forming a thin film. Collagen film has been proven to be a promising vehicle [21].
A preservative-free hydroxypropyl cellulose stick-shaped tablet is commercially available. This device is designed to continuous- release artificial tear. It is used to treat dry ocular disorders. Merck, Sharp, and Dohme created it in 1981 [21].
Commercial filter paper strips made in sodium fluorescein and rose Bengal dyes. These dyes used in diagnostic procedures to reveal dry eye conditions, herpes simplex infections and corneal injuries [21].
Semisolids is a wide range of topical ophthalmic delivery methods. These substances can be divided into simple bases and compound bases. A single continuous phase is referred to as a simple base. These include viscous gels made from polymers like PVA, Carbopol and white petrolatum, as well as lanolin. Most compound bases are biphasic. Ointments disperse solid drug in a suitable vehicle base are the most widely used in semisolid preparation [21].
Ocular iontophoresis, Vesicular systems, Mucoadhesive dosage forms, Particulates, Ocular penetration [21]. Solutions, suspensions, emulsions, hydrogels, inserts, in-situ gels, liposomes, injections etc. are various excipients. The various excipients used in the ophthalmic drug delivery system (Figure 1) [23].
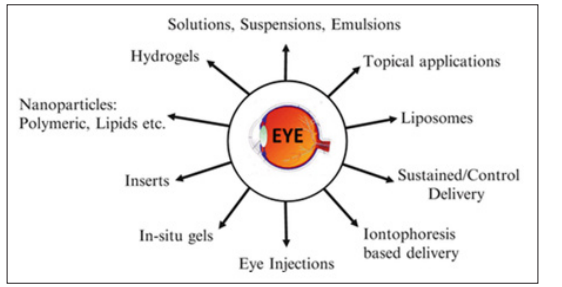
Figure 1: Types of Ophthalmic Drug Delivery System
The challenge is achieving drug concentrations at the active site at the right time. The treatment of ophthalmic drug delivery system is necessary. The corneas anatomy, physiology, and barrier function work together to prevent rapid drug absorption. The drug is maintained at therapeutic levels in the tear film or at the site of action for eye instillation [24]. The diffusion and absorption of topically applied drugs in the precorneal and corneal spaces [25]. However, this can cause negative side effects and cell damage to the ocular surface [24]. Conventional ophthalmic dosage forms have low ocular bioavailability. These dosage forms are caused by precorneal restrictions [25]. The primary obstacles are solution drainage, lacrimation, tear dynamics, dilution, turnover, conjunctival absorption, ineffective absorption, temporary residence time and relative corneal epithelial membrane impermeability. The drug can only be absorbed in very small amounts through the eyes due to these physiological and anatomical restrictions [24]. For topical formulation to be clinically effective, lipophilicity and hydrophilicity must be balanced with longer contact times [26].
The active ingredient is absorbed by both the cornea and conjunctiva. It flows into the lacrimal sac through the upper and lower canaliculus. (Figure 2) [25].
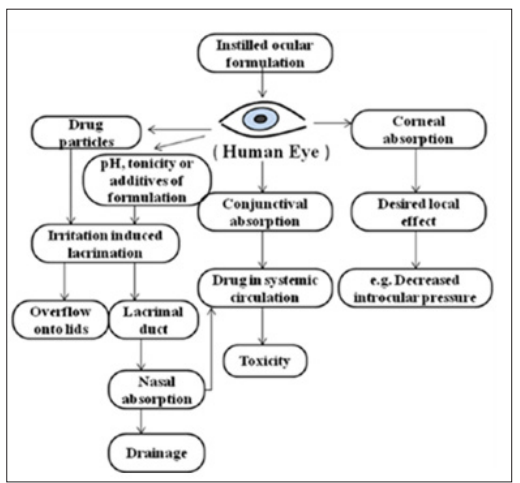
Figure 2: Flowchart on the Challenges in Ophthalmic Drug Delivery System
There are two categories of methods that have been tried to extend the therapeutic effect of ophthalmic drugs therapeutic bioavailability and duration [24].
The ophthalmic bioavailability improved by conventional methods [24]. Modern methods improve ophthalmic bioavailability of drugs and regulate ophthalmic drug release [28]. Ophthalmic drugs are administered by intravitreal injection, iontophoresis, subconjunctival injection and periocular route [24]. There are two types of approaches used to enhance ophthalmic drug delivery system. Increasing corneal permeability is one method to improve ophthalmic drug bioavailability after topical administration. The second method for maintaining and managing the sustain and control release drug delivery system (Figure 3) [29].
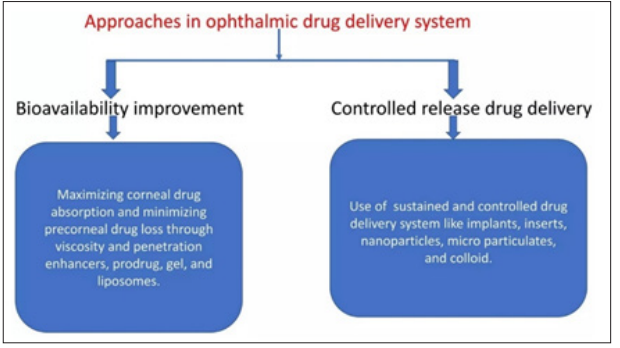
Figure 3: Flowchart on the Various Approaches in Ophthalmic Drug Delivery System
Endothelial cells make up the anterior blood-eye barrier. This barrier restricts the entry of hydrophilic medications and plasma albumin into the aqueous humor. Retinal pigment epithelium (RPE) and retinal capillary walls provide the posterior barrier between the blood supply and the eye. The choroids vasculature has a large blood flow and leaky walls in contrast to retinal capillaries. The RPE and retinal endothelia restrict drug distribution into the retina after they have entered the choroidal extravascular space [3].
After instillation, the lacrimal fluid flow removes the compounds from the ocular surface. The lacrimal turnover rate is only about 1μl/min. The extra volume of instilled fluid is quickly transported to the naso-lacrimal duct within minutes. Systemic absorption is the cause of ineffective drug removal. The systemic absorption is possible once the solution has passed through adjacent blood capillaries and into the nasal cavity or straight from the conjunctival sac [3].
The corneal epithelium limits the absorption of drugs from tears. Tight junctions are formed by the corneal epithelial cells. It restricts the permeation of paracellular drugs. Lipophilic drugs have a higher permeability in the cornea. Surface area of the conjunctiva has nearly 20 times larger. Its epithelium is generally leakier than the cornea [3]. Blood-ocular barriers protect the eye from xenobiotics in the blood stream. These barriers consist of blood-aqueous and blood-retina components (Figure 4) [3].
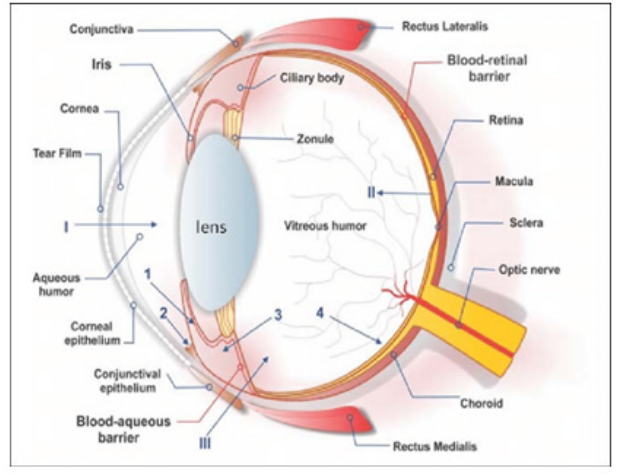
Figure 4: Barriers in Ophthalmic Drug Delivery System
Incorporation of drug particles or additives must not affect the transparency of the formulated contact lens. These lenses play a key role in ophthalmic drug delivery [25]. Innovative methods have made it possible to create and include materials in contact lenses that displayed good transparency [30]. Contact lenses, loaded liposomes and microemulsions developed using molecular imprinting and supercritical solvent approaches demonstrated superior transparency techniques [30].
The human eye’s blood flow is inadequate for effectively removing carbon dioxide and providing sufficient oxygen. It primarily depends on exposure to air for oxygen supply [25]. The prepared eye lens must allow free flow of oxygen. Insufficient oxygen transport can cause serious side effects [31].
The Tg of contact lenses is changed due to drug loading processes or additive additions. Numerous studies indicate that contact lenses produced using various methods do not significantly impact Tg [32]. The change in Tg upon βCD addition was found to be insignificant. The degree of cross-linking and network stiffness of the hydrogels are not significantly affected by the grafted βCD [33]. As a result, it is generally feasible to load drugs into most contact lens fabrication techniques without changing Tg [25].
Most of the tear surfaces water will be repelled by hydrophobic polymers. This interferes with the tear flow. This causes an albumin film to form on the lenses. It reduces the effectiveness of contact lenses over time. It also increases the risk of infection and inflammation [34]. A contact lens surface must be made hydrophilic if it is highly hydrophobic. This modification in the surface’s morphology can be achieved by doping the polymer or treating its surface [25]. The stability of the pre-lens lacrimal fluid is crucial for the wettability of soft contact lenses. The assessment can be done by determining the contact angle. The wettability of pHEMA increased copolymerization with a high concentration of glycidyl (GMA) methacrylate but decreased with the addition of βCD [33]. However, extended drug delivery can be achieved with contact lenses that use β-CD-loading. The contact angle was not significantly impacted by the application of timolol maleate and acetazolamide. It was used by the supercritical solvent impregnation technique [32]. The absence of lens surface wettability is indicated by theoretical contact angles greater than 90º [35].
The permeability of the lens is directly influenced by its water content. The percentage of water weight in the lens causes the permeability to increase linearly. Lenses can absorb a lot of water and make them extremely hydrophilic. Soft contact lenses provide superior permeability and extended wear without causing ocular discomfort. Increasing the water content will eventually lead to decrease in the strength of the polymer. Polymer making is difficult to increase permeability. As a result, the lens may be torn or scratched. Additionally, the cornea is less protected by the softer lens. Lenses with lower water content release more water into the tear film later. The number of hydrophobic drugs released from a lens does not seem to be linked to its water content [36]. Hydrophobic drugs partition into silicone-rich phases with the silicone content of gels influencing the drugs partition coefficient [25].
Numerous polymers are available that meet the requirements for ophthalmic administration [37]. According to their source, they can be divided into natural, semi-synthetic and synthetic polymers [38]. Most polymers are biodegradable by enzymes or water and non-biodegradable prototypes. Eudragit® is a family of polymethacrylates. It has also emerged as a promising alternative [39].
Hybrid Polymer Carriers for Ophthalmic Drug Delivery System Polymers for ODDS are available in a wide range of materials. Colloidal systems loaded with drugs work better than drug solutions but there are still problems that need to be addressed.
Water-soluble drugs are best encapsulated in hydrophilic polymers. These drugs are unable to sustain release of the drugs in an aqueous biological environment. On the other hand, hydrophobic polymers enable not only hydrophilic polymers [37].
Clarity can be assessed by examining the formulations under black and white lighting conditions [40].
Gelling was taken one drop of the formulation into a vial containing 2.0 ml of freshly prepared simulated tears. It was observed visually [41].
Irritation tests are designed to evaluate the potential use of ophthalmic products. It causes eye irritation before marketing. According to the Draize test, the amount of substance applied to the eye is usually 100 μl [3]. The drug was placed in the lower cul-de-sac for 1 hour, 24 hours, 48 hours, 72 hours and one week after administration. It was observing various criteria. The rabbit was regularly observed for redness, swelling and tearing [42].
Isotonicity is an essential quality of ophthalmic preparations. It avoids tissue damage and ocular irritation [3]. All ophthalmic preparations are tested for isotonicity. The mixtures are compared to commercially available standard ophthalmic formulations after being viewed under a microscope at a 45X magnification [43].
The consistency, hardness and adhesion of in situ gel were evaluated by a texture profile analyzer. It indicates the gel durability and ease of use in vivo. The higher adhesion value of the gel maintains tight contact with the mucus surface [44].
In-vitro diffusion using a specially designed glass cylinder with a semi-permeable cellophane membrane/dialysis membrane for open flow. The donor and receiver compartments separated by a sandwich of cellophane membrane in simulated tear fluid [3].
The corneal permeation membrane is investigated using goat corneas. A portion of the corneal scleral tissue measuring 5-6 mm is removed and washed with cold saline. The washed corneas kept in cold freshly prepared solution of pH 7.4 in tear buffer. The Franz-diffusion cell is used for the study [3].
In-vivo scintigraphy is the assessment of ocular retention time. Due to physiological differences between rabbits and humans, human volunteers are preferred for this study. The blinking rate is a commonly recommended animal model for evaluating ophthalmic formulations [45].
The prepared gel preparations were stored for six weeks at ambient temperature in the working area of 25°C to 28°C, 4 ± 1°C in the refrigerator and 37 ± 2°C in the incubator. Gels are examined for color, consistency, active ingredient content and degradation rate constant (K). The samples were put through stability studies to determine the shelf life. Selected sterilized formulations kept at 4 ± 1°C in the refrigerator, 37 ± 1°C at room temperature and 45 ± 1°C at an extreme temperature of three months. These samples are then analyzed every seven, fourteen, forty-two, sixty and ninety days. The formulations are evaluated for drug content, clarity, pH, sol-gel transition, rheology, in-vitro drug release and sterility using UV Spectrophotometer [3].
The amount of an ophthalmic drug that can be absorbed is severely constrained by physiological limitations. The relatively impermeable corneal barrier regulates ocular absorption. The three membrane layers act as the main absorptive barriers. Ion transport is inhibited by the lipophilic cellular layers of the epithelium that faces the tears. The corneal epithelium acts as a selective barrier for small molecules and stop the paracellular route of macromolecular diffusion. 90% of the cornea is made of the highly hydrophilic stroma layer that lies beneath the epithelium. The normal hydration of the cornea is maintained by the corneal endothelium. The more lipophilic drugs are the more difficulty in penetrating the stroma. The epithelium is more resistant to drugs are more hydrophilic. The stroma and endothelium have a lower level of resistance. The drugs physicochemical properties influence its route and rate of permeation through the corneal membrane in the cul-de-sac [24].
The numbers refer to following processes [46].
Drug diffusions occur in site of action through media as well as tissues with different forms of consistency from liquid aqueous humor to solid lens [47]. Drugs are absorbed topically and then enter the aqueous humor through the absorption process. Absorption can impact on ophthalmic drug solubility in tears and surface permeability. The conjunctiva and sclera are more permeable to hydrophilic drugs [48]. The amount of medication reaches the aqueous humor is referred to as bioavailability in ophthalmology. The intraocular tissues where the drug is further dispersed and transmitted. Topical ophthalmic medications may be accessible depending on several factors. Drugs can build up in every ocular tissue. Large conjunctival surfaces and nasal mucosa allow some topical medications to reach the eye before they are absorbed. When a substance crosses the blood-ocular barrier or occurs during aqueous humor turnover, the eye is eliminated [49]. The ophthalmic route frequently employs antibiotics applied as a solution or an ointment. Antibiotics are also used to implants, contact lenses, colloidal and in situ gelling systems (Figure 5) [2].
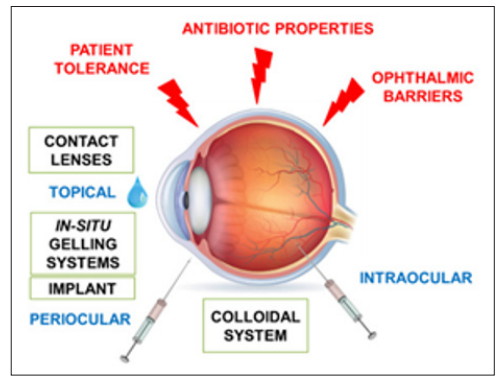
Figure 5: Mechanism of Ophthalmic Drug Delivery System
Antibiotics are a group of drugs commonly used in ophthalmology for many ocular diseases. Penicillins, aminoglycosides, fluoroquinolones, tetracyclines and fluoroquinolones are a few examples of antibiotics frequently used to treat ocular infections [18]. Topical, subtenon, intraocular, or subconjunctival applications to the eye are possible ways to deliver antibacterial therapies. The new methods of ophthalmic drug delivery systems are nanoemulsions, liposomes, microemulsions, niosomes, nanoparticles and in situ gelation systems [2].
|
Brand Name |
Active Drugs |
Dosage Form |
Manufacturer |
Uses |
|
Acivir eye |
Acyclovir |
Ointment |
Cipla Limited |
Eye infection |
|
Betnisol N |
Betamethasone |
Eye drop |
GSK Pharma |
Eye infection |
|
Chloromycetin B |
Chloramphenicol palmitate |
Ointment |
Century Pharmaceuticals Limited |
Bacterial eye infection |
|
Ciplox |
Ciprofloxacin |
Eye drops |
Bayer HealthCare Pharmaceuticals Limited |
Eye infection and conjuctivitis |
|
Dexcin |
Dexamethasone |
Eye drop |
Syntho Pharmaceuticals Private Limited |
Eye infection |
|
Dichol |
Carbachol |
Sterile solution prefilled syringes |
Dahlgren Pharma |
Ophthalmic surgery |
|
Geltear |
Carbomer |
Bioadhesive gel |
Bausch & Lomb Incorporated Limited |
Lubricant, burning, irritated a dried eye |
|
Brand Name |
Active Drugs |
Dosage Form |
Manufacturer |
Uses |
|
Ocupol |
Polymixin-B |
Eye drops and ointment |
Centaur Pharmaceuticals Private Limited |
Bacterial infection, corneal ulcer |
|
Pred forte |
Prednisolone acetate |
Suspension |
Allergan Pharma |
Anti-allergenic and anti- inflammatory |
|
Refresh classic |
Artificial tear fluid |
Single use vials |
Allergan Pharma |
Relieves dry and irritated eye |
|
Refresh tears |
Hydroxy propyl methyl cellulose |
Eye drops |
Allergan Pharma |
Dryness of eye |
|
Restasis |
Cyclosporine |
Emulsion |
Allergan Pharma |
Dry eye |
|
Timol Xe |
Timolol maleate |
In-Situ gel |
Bausch & Lomb Incorporated Limited |
Dried eye and keratoconjuctivitis |
The factors limiting the absorption of topical products in ophthalmic formulations are high tear turnover rate, high tear drainage, reflex tear production, tear film barrier and drug loss from rapid blinking. The duration of surface action increases as the bioavailability of ophthalmic drug will increase [51]. There are several barriers that need to be overcome in the development of ophthalmic products [52].
Eye drops are the most popular dosage forms. After administration, there is a minor burning sensation that causes lacrimation and cell desquamation [2]. Ocular bioavailability can be improved by increasing corneal penetration and residence time on the ocular surface [53].
Ointments are sterile, semi-solid and homogenous formulations designed for ophthalmic application. Non-aqueous excipients must not irritate the eyes when used in this mixture. This method slows the drugs excretion through the tears. By extending surface time residence, it also lengthens the corneal residence time. It is advisable to apply ointment in the evening to treat blurry eyesight [2].
Hydrogel can extend drug retention time and maintain drug release. These formulations have low ocular bioavailability and require frequent dosing [52]. The blinking and flushing of tears can be resisted by hydrogels. Hydrogels have low stability and/ or short half-lives in the vitreous fluid [2].
A co-surfactant may occasionally be used to stabilize the oil- in-water dispersion in this system. This substance enhances the solubility and dissolution efficiency of drugs that are less water- soluble. However, this form has certain limitations [2].
Microspheres and Nanoparticles are the most promising drug carriers for ophthalmic applications. The much slower rate of ocular particle elimination causes a significant increase in drug absorption in the eye. Nanoparticles may be very comfortable ophthalmic prolonged action delivery systems [50]. However, albumin microspheres cause unwanted effects in the eyes [54]. The ophthalmic drug delivery development systems to treat cystinosis has been slower than for other ocular disorders. The stability of cysteamine and the rarity of the condition with connected to the challenges (Figure 6) [51].
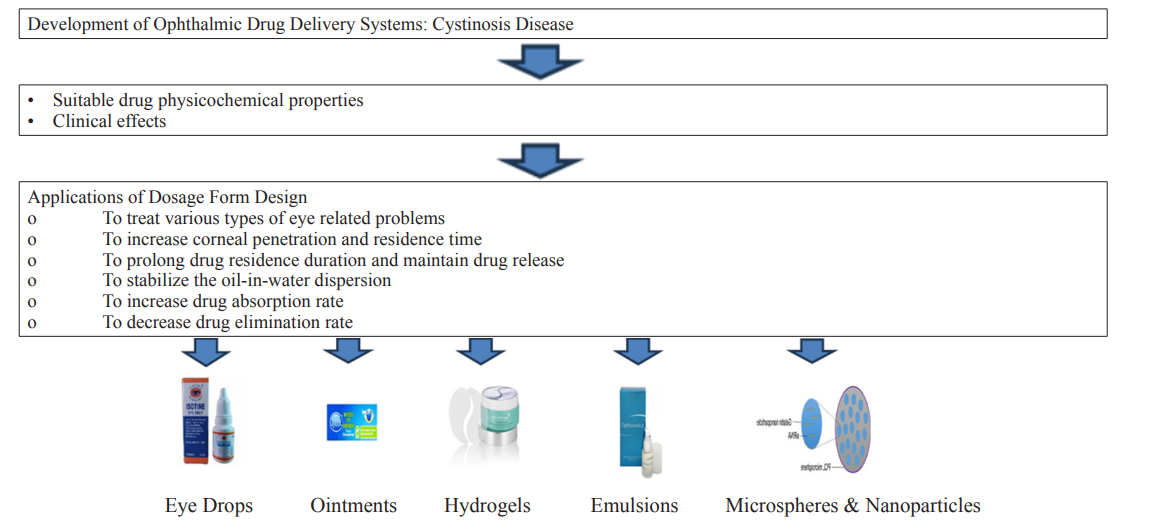
Figure 6: Flowchart on the Application of Ophthalmic Drug Delivery System
The Vision Loss Expert Group (VEEG) revealed 90% of the 1.1 billion people worldwide suffering from vision loss in low and middle-income countries. Cross-linked polyacrylic acids are present in two preparations. NyoGel contains timolol maleate from Novartis and Pilogel contains pilocarpine hydrochloride from Alkon Laboratories [55]. These two types of preparations exhibit mucoadhesive properties [56]. There are several ways to administer substances to the eyes, including solutions, suspensions, gels, ointments and implants [57]. These methods of medication delivery through the eyes are nearly exclusively used [58]. It is utilized to treat nearby ophthalmic infections [59].
|
Brand Name |
Generic Name |
Dosage Form |
Manufacturer |
Indications |
Side Effects |
|
A-Phenicol |
Chloramphenicol |
Eye drop |
ACME Laboratories Ltd. |
Antibacterial infections |
Blurred vision, burning, itching, rashes and eye redness |
|
Acicaft |
Alcaftadine |
Eye drop |
ACI Limited |
Itching associated with allergic conjunctivitis |
Eye irritation, burning, eye redness and eye pruritus |
|
Acicot |
Dexamethasone |
Eye drop |
ACI Limited |
Non-Infectious form |
Burning sensation, cataract and corneal softening |
|
Alacot |
Olopatadine hydrochloride |
Eye drop |
Square Pharmaceuticals Ltd. |
Allergic conjunctivitis |
Blurred vision, dry eye, burning, sinusitis |
|
Cloram |
Chloramphenicol |
Eye ointment |
Ibn Sina Pharmaceuticals Industry Ltd |
Conjunctivitis, blepharitis, corneal ulcer |
Rashes, fever and angioedema |
|
Aprocin |
Ciprofloxacin |
Eye ointment |
Aristo Vision |
Antibacterial infections |
Eye irritation and burning sensation |
|
Ciprocin |
Ciprofloxacin |
Eye drop |
Square Pharmaceuticals Ltd. |
Antibacterial infections |
Rashes, itching, eye redness |
|
Opthaflox |
Lomefloxacin hydrochloride |
Eye drop |
Drug International Ltd. |
Antibacterial infections |
Eye irritation, redness, itching |
|
Optimox |
Moxifloxacin hydrochloride |
Eye drop |
Aristo Pharma Ltd. |
Antibacterial infections |
Eye discomfort, dry eye,burning sensation
|
|
Vista |
Ofloxacin |
Eye drop |
Aristo Pharma Ltd. |
External ocular infections |
Eye irritation, burning, eye redness, itching |
|
Povisep |
Povidone-iodine |
Eye drop |
Jayson Pharmaceuticals Ltd. |
Dry eye conditions and antibacterial infections
|
Local irritation, hyper sensitivity |
|
Clofenac |
Diclofenac sodium |
Eye drop |
Square Pharmaceuticals Ltd. |
Eye pain, inflammation and sensitivity to light after surgery |
Eye redness, irritation,blurred vision, b urning, dry eye or itching
|
|
Diclofen |
Diclofenac sodium |
Eye drop |
Opso Saline Ltd. |
Eye pain, inflammation and sensitivity to light after surgery |
Eye redness, irritation,blurred vision, burning, dry eye or itching
|
|
Aristophen |
Chloramphenicol |
Eye ointment |
Aristo Vision |
Antibacterial infections |
Allergic reactions, itching, irritation, eye redness |
|
Emodol |
Ketorolac tromethamine |
Eye drop |
Jayson Pharmaceuticals Ltd. |
Eye pain, inflammation and sensitivity to light after surgery |
Eye irritation, burning, blurred vision, eye redness |
|
Etorac |
Ketorolac tromethamine |
Eye drop |
Incepta Pharmaceuticals Ltd. |
Ocular itching, inflammation, allergic conjunctivitis |
Allergic reactions, burning, blurred vision, eye dryness |
|
Levorax |
Levocetrizine dihydrochloride |
Tablet |
Popular Pharmaceuticals Ltd. |
Antibacterial infections |
Headache pain, dizziness, nausea, constipation, dialarhoea |
Recent Developments in Ophthalmic Drug Delivery System Most conventional ophthalmic dosage forms are simple. Water- soluble medications provided an aqueous solution. Water-insoluble medications applied as an ointment or an aqueous suspension [21]. The main drawbacks of conventional dosage forms are poor ophthalmic drug bioavailability, pulse drug entry, systemic exposure due to nasolacrimal duct drainage and inefficient drug transport to posterior ocular tissue. Ocular anatomical and physiological restrictions are the blood-ocular barriers high efficiency, the relative impermeability of the corneal epithelial membrane, tear dynamics and nasolacrimal drainage, all contribute to poor ocular medication absorption [60]. Only 1% or less of the topical dose is usually absorbed by the cornea. It migrates to the anterior area of the eye [61]. Eye drops often have pharmacokinetic characteristics are called pulse entry. High drug concentration in tears followed by a rapid decline, increases the risks of potential toxicity and indicates the need for frequent dosing. The challenge overcoming toxicity due to high initial concentration without frequent dosing especially for potent drugs [21]. Nasolacrimal drainage is a significant factor in precorneal drug loss. It results in poor ocular bioavailability. Topical administration of ophthalmic drug route is the primary entry point into the circulatory system. The systemic exposure through nasolacrimal drainage can lead to high toxicity. Timolol has been linked to systemic toxicity in the ophthalmic solution after topical administration [62]. Timolol is hindered by poor ocular bioavailability. The blood-retinal barrier restricts the intravenous route in posterior ophthalmic drug delivery. Intravitreal injection is the most effective for ophthalmic drug delivery method. It also poses a high risk of complications [21]. Some recent advances in topical ophthalmic drug delivery are iontophoretic, in situ gelling systems, dendrimers, penetration enhancers, lipid emulsions, ocular inserts, stimuli-insensitive hydrogels, muco-adhesive polymers and site-specific systems. The goal of these efforts is to improve ophthalmic drug bioavailability for extended periods or facilitating transcorneal penetration. Traditional eye drops bottles administer 95% of medicines [63]. Research on improving ocular contact time of solutions initially involved incorporating polymers into an aqueous medium. The solutions viscosity increased, thereby decreasing the drainage from the eye. Methylcellulose increases the pilocarpine solutions viscosity from 1 to 100 cps. It decreased the solution drainage rate constant by ten times and increased in the aqueous humor by two times. The optimal viscosity for ophthalmic drug absorption is suggested to be 12- 15 cps [63]. Natural polymers have been investigated as viscosity inducers with prolonged residence time and duration of action. These polymers were observed at 1% pilocarpine [64]. The bio adhesive polymers can enhance the residence time in the precorneal region [65]. OptiMyst and Versidoser dispense medication as a mist or multidose in small volumes, reducing blink and lachrymation thresholds [63]. A new ophthalmic method uses a temporary applicator under the lower eyelid. It transports an ionized drug to ocular tissues by using a low electrical current [66]. Recently, advanced technology using nanocarriers is being explored to improve ophthalmic drug delivery [67]. These systems claimed to offer extended ocular surface residence time, thereby reducing the impact of natural eye clearance systems [68]. Injections and sustained release implants are examples of advanced subcutaneous delivery techniques. These techniques carry a higher risk of infection, internal ocular hemorrhage and retinal damage [69].
Current Statuses of Ophthalmic Drug Delivery System Various ophthalmic drug delivery systems are currently available in the market or undergoing clinical trials for sustained drug release. Most ophthalmic drugs are used for treating long-term eye diseases [73]. Ophthalmic drug delivery systems are crucial for treating macular degeneration, viral infections, glaucoma, ocular inflammations, dry eye syndrome and retinal degenerations [74]. Current ophthalmic drug delivery systems with lower bioavailability and invasive nature, pose challenges for novel technologies to improve treatment for ocular disorders. Eye drops are expected to be the most popular treatment for ocular disorders over the next 20 years. The low bioavailability of eye drops at the target tissue presents a challenge. Researchers are actively working to enhance the in vivo performance of conventional formulations. Advances in nanotechnology, new techniques, devices and applications in drug delivery are becoming increasingly popular among ophthalmology researchers. Drug molecules are administered by invasive, non-invasive or minimally invasive methods by being encapsulated in nanosized carrier systems or devices [53]. Controlled drug delivery using nano formulations can enhance the bioavailability of drugs in anterior tissues. All current treatments for ocular disorders are invasive in nature. Retinal detachment, bleeding and patient discomfort can result from repeated intravitreal injections. Ophthalmic drug delivery scientists are working on developing non-invasive and sustainable systems for the posterior eye segment. New noninvasive drug delivery systems are needed to overcome ophthalmic barriers, sustain drug release and maintain effective drug concentrations in the eye [5]. Current studies highlight the primary barriers that cause poor drug absorption and bioavailability [75]. Drug bioavailability is restricted by efflux transporters for steroids, antibiotics and anticancer medications [76]. Studies suggest conjugating drugs to dendrimers to bypass efflux transporters, increasing drug solubility and bioavailability [13]. Propranolol is a calcium channel blocker and substrate of the p-glycoprotein efflux transporter. It was conjugated to lauroyl-G3 dendrimers [1]. The conjugation demonstrated improved drug solubility [77]. Ocular nano-carrier DDSs have shown significant progress in drug stability, solubility, corneal permeability, retention time, bioavailability and efficacy. It is needed on their mechanism of action, quality control and safety evaluation. The vehicles utilized for nano-carrier DDSs require further evaluation. The toxicity of excipients and pollutants has been assessed in vitro. The inflammatory reaction has been assessed in vivo [78]. The size of the mouse lens differs significantly from humans. The main differences among them are drug permeability from the cornea to the retina. The challenge is achieving efficient drug delivery to the posterior segment using non-invasive methods. Current research trends focus on the design and synthesis of new support materials or the use of multiple methods to prepare composite systems [79].
The NODS® is a water-soluble and drug-loaded film administration [55]. The device consists of a medical flag. Medical flag attached to a paper-covered handle by a short and thin membrane. Water-soluble polyvinyl alcohol (PVA) is used to make each component [80]. Each device is individually packed and irradiated with gamma radiation. To apply the flag, it is applied to the bottom conjunctival sac [55]. The membrane then dissolves and releases the drug into the lacriminal fluid.
When tested in humans, NODS® increased the bioavailability of pilocarpine by 8-fold [21]. New ophthalmic drug delivery systems are nano emulsions, liposomes, microemulsions, niosomes, in situ gelation systems and nanoparticles. These methods can be used for both hydrophilic and lipophilic medications [2]. The system can be targeted to a specific location and can be managed through various methods. In situ gelling systems with suitable excipients can prolong precorneal residence time and reduce drug loss due to tear. Different polymers, planning strategies and compositions allow nanoparticles to address mucoadhesive, topical, periocular or intraocular administration requirements. The various administration requirements result in stable, effective and nonirritating formulations for patients [2]. Polymers with different channel widths in the matrix control the rate of drug delivery and remain effective for a longer time. Soft contact lenses are more popular for increasing bioavailability characteristics [81]. Some approaches are adding binding agents by molecular imprinting and entrapment of vesicular systems into the lens matrix [16]. These approaches have been proposed to enhance ophthalmic drug delivery system [25]. NODS do not contain preservatives [82]. A novel treatment strategy in ophthalmology is cyclodextrins. Cyclodextrins is used to increase the drug solubility in solution and corneal permeability. Collagen shields are a modern continuous- delivery framework for drugs. It provides high and sustained levels to the cornea despite resilience issues [83].
Future Prospects of Ophthalmic Drug Delivery System Infections, glaucoma, cataracts, dry eyes and other anterior segment diseases can all lead to blindness or impaired vision over time. Eye drops are commonly used to treat certain conditions, but their effectiveness is limited by various factors. The use of films, hydrogels and implants for drug delivery has the potential to enhance the duration of ocular residence. These methods can potentially hinder vision and cause discomfort to patients. The safety of ocular tissue may be compromised due to prolonged contact with the carrier material in implants. Nanoocarriers have shown promise in preclinical studies and clinical trials for safer and more efficient ophthalmic drug delivery. Nanocarrier targeting the posterior region is challenging, necessitating a delivery method that can bypass ocular barriers for therapeutic delivery [20]. The main focus going forward will be to achieve non-invasive sustained drug delivery for ocular diseases. Improved comprehension of physiological barriers, multicompartmental pharmacokinetics and tissues under normal and pathological conditions would accelerate future research in this area. The optimal system minimizes systemic exposure and prolongs drug concentration at the target tissue is ideal. The system should be simple and comfortable [24]. Chronic retinal disorders are currently treated using injectable formulations. AMD requires intravitreal injection for anti-VEGF treatment. It requires repeated administration and expert supervision. Injectable nanomedicine can bypass vitreous clearance and deliver drugs directly to the retina. The nanomedicine can maintain its concentration in the vitreous region, providing extended salutary benefits to the targeted site. Nanomedicines can be administered intravenously, reducing dose, frequency and invasiveness. Thus, reducing medical burden for patients and healthcare professionals [20]. Combining technologies is a viable approach to overcome the limitations of individual technologies. Liposomes and nanoparticles in droppable gels coated in bio adhesive polymers have been reported [24]. The delivery of nanocarriers can cause increased aggregation and vision disturbance. The study found that particles reduced aggregation chances, improved drug release and loading are crucial aspects. The ophthalmic drug delivery of nanoparticles is hindered by various factors. The main focusing points of the research are the application of controlled drug release from implants, nano wafers, 3D printed hydrogel technology and nano-drug delivery systems to retinal diseases. Nanomedicine must be safe, scalable and reproducible in order to achieve commercialization [20]. Advances in science and technology are significantly accelerating progress in this field. The creation of smart technologies to improve ophthalmic drug delivery could be facilitated by developments in nanotechnology and biomaterials science [24]. Natural and biodegradable polymers are generally safe for ophthalmic drug delivery. In this system, extensive toxicity studies are required for commercial approval. Nanomaterial preparation techniques have been explored for large-scale production. It is simpler, more feasible and regulatory-accepted methods are needed [20].
Nanotechnology can enhance treatment effectiveness and offer novel features in ophthalmic drug delivery systems. The Ophthalmic Drugs Market is estimated to be worth $25.03 billion worldwide in 2017. It’s expected to make a total of $34.52 billion in revenue by 2024. The projected annual compound growth rate (CAGR) of the market is estimated to be 4.7% from 2018 to 2024 [20]. The future potential of ocular new drug product applications is evident [24].
After reviewing the mentioned literature, we can know that the pharmaceutical and medical sciences face significant obstacles in the ophthalmic drug delivery methods [2]. An ophthalmic drug delivery system is used to treat various ocular diseases and vision related conditions. Formulators face difficulties in obtaining the eyes defenses without resulting in long-term tissue damage [3]. The primary challenge in treating ocular problems is maintaining an effective medicine concentration at the therapeutic target for extended periods [4]. The cause of this is the quick drug clearance caused by blinking and lachrymation [10]. The major cause of irreversible blindness is retinal disease [11]. A few examples are age-related macular degeneration (AMD), proliferative vitreoretinopathy (PVR), diabetic macular edema (DME), endophthalmitis, cytomegalovirus (CMV), retinitis and retinitis pigmentosa [12]. Topical, systemic, periocular and intravitreal barriers are the main administration route on eye [4]. The various barrier properties overcome the eyes defenses without causing permanent tissue damage [3]. The most popular technique for treating ocular diseases is topical route of administration [6]. The primary obstacles are solution drainage, lacrimation, tear dynamics, dilution, turnover, conjunctival absorption, ineffective absorption, temporary residence time and relative corneal epithelial membrane impermeability [24]. Several new ophthalmic drug delivery methods have recently been developed. These include hydrogels, polymer micelles, nanoparticles (NPs), implants, microneedles, ion electrophoresis, ultrasound and more [4]. One of the most important and promising ophthalmic drug delivery methods is nanotechnology [1]. Some approaches are adding binding agents by molecular imprinting and entrapment of vesicular systems into the lens matrix [16]. Various elimination mechanisms make it difficult for conventional ophthalmic formulations to adequately deliver drugs to the retinal tissues [13]. Eye drops address complicated penetration barriers corneal, blood-aqueous (BAB) and blood-retinal (BRB), nasolacrimal drainage, protein binding, systemic absorption and enzymatic degradation [14]. The most common ophthalmic control drug delivery systems are gels, ointments, liposomes, micro- and nanoparticles, microspheres and ocular mini tablets (MT) or films [17]. Numerous experiments were conducted to create effective ophthalmic drug delivery systems (ODDS). ODDS enhances the duration of treatment, targeting and adherence [19].
The World Health Organization estimates that one person worldwide loses their vision every five seconds. Globally, 1.3 billion people suffer from vision problems [20]. Over the past few decades, the dosage formulations used today have improved. Ocular dosage forms must be sterile, secure and free of the patient’s allergies [2]. Cross-linked polyacrylic acids are present in two preparations. NyoGel contains timolol maleate from Novartis and Pilogel contains pilocarpine hydrochloride from Alkon Laboratories [55]. These two types of preparations exhibit mucoadhesive properties [56].
Some recent advances in topical ophthalmic drug delivery are iontophoretic, in situ gelling systems, dendrimers, penetration enhancers, lipid emulsions, ocular inserts, stimuli-insensitive hydrogels, muco-adhesive polymers and site-specific systems. The goal of these efforts is to improve drug bioavailability by delivering drugs to the eye for extended periods or facilitating transcorneal penetration [63]. Research on improving ocular contact time of solutions initially involved incorporating polymers into an aqueous medium [84]. Current ophthalmic drug delivery systems with lower bioavailability and invasive nature, pose challenges for novel technologies to improve treatment for ocular disorders [53]. All current treatments for eye disorders are invasive in nature. Retinal detachment, bleeding and patient discomfort can result from repeated intravitreal injections [5]. The main focus going forward will be to achieve non-invasive sustained drug delivery for ocular diseases. Improved comprehension of physiological barriers, multicompartmental pharmacokinetics and tissues under normal and pathological conditions would accelerate future research in this area. The optimal system minimizes systemic exposure and prolongs drug concentration at the target tissue is ideal. The system should be simple and comfortable [24]. The Ophthalmic Drugs Market is estimated to be worth $25.03 billion worldwide in 2017. It’s expected to make a total of $34.52 billion in revenue by 2024. The projected annual compound growth rate (CAGR) of the market is estimated to be 4.7% from 2018 to 2024 [20]. The future potential of ophthalmic new drug product applications is evident [24]. To reduce this problem would likely be more pseudoplastic than the materials currently in use and more hydrophilic [85].
Most researchers are taking on challenges to address a variety of issues developing the field of ophthalmic drug delivery. These challenges could address a range of problems in the emerging field of ophthalmic drug delivery. Contemporary techniques have enabled improved treatment efficacy of the ophthalmic route. Both biological and non-biologic limitations present difficulties for the effective administration of ophthalmic drugs. Innovative methods have been recently developed to address challenges in drug delivery to the eye. These methods overcome the limitations of conventional ophthalmic preparations. Advances in the field of ophthalmic drug delivery have been made recently with load control and prolonged release. Modern methods have made it possible to increase the therapeutic effectiveness of the ocular route. The most recent advancements in drug delivery are encouraging. The potential applications of iontophoresis for a range of ocular diseases have become possible due to its promising outcomes in corneal cross-linking. In-Situ gelling system is a promising method for extending precorneal resident time, enhancing drug delivery, improving ocular bioavailability, reducing systemic absorption and toxicity. Liposomal drug delivery systems are highly advantageous. These types of drug delivery systems can effectively entrap both hydrophilic and hydrophobic drugs. The use of biodegradable and water-soluble polymer formulations may result in more acceptable and superior ophthalmic drug delivery systems. Therefore, a polymer model with physical retention capabilities is required to increase the drug residence time at the corneal surface and preserve visual function. To reduce blink noise, such systems would likely be more pseudoplastic than the materials currently in use and more hydrophilic. Future ophthalmic drug delivery innovations aim to improve eye health, patient compliance and manage ocular diseases by enhancing patient compliance and achieving superior results.
