Author(s): K Sreevani* and VV Anierudhe
Industrial heavy metal pollution and other effluents from chemical industries are of great concern for the ecosystem. Due to this, toxic waste water is released into the environment causing severe heavy metal pollution and also affects our health. The physicochemical methods are expensive and these results in the incomplete removal of metal ions. As a result, other alternative methods such as algae bioremediation are taken into account which have the ability to absorb metal ions and this very idea can be used in the treatment of wastewater. They absorb metal ions on the cell surface and in the intracellular ligands. Before releasing algae into waste water, they are treated with pretreatment. Various pretreatment improves the enhancement of metal sorption in algae. The most suitable and economic method for cultivating algae biomass is the calcium chloride pretreatment. When considering algae, there are various strains and hence, selection of strain is highly important. This happens by the understanding of bio sorption mechanisms, increasing the number of surface groups with genetic manipulation of algae and creating them for economic feasibility. In brown algae, the order Laminariales and Fucale species are considered as efficient groups of algae while bio-sorption is considered, because their cell wall is very rich in extracellular polymers and polysaccharides. Considering these parameters in the significant development of low-cost algae cultivation and an eco-friendly solution for the pollution caused by chemical industries, this work concentrates on the bioremediation process for the reduction of heavy metal pollution.
The heavy metal industry production and textile industries play a major role in the economy but at the same time they release heavy metal pollution into the natural environment. Metal ions like lead, copper, cadmium, zinc, and nickel create toxic impurity in water and natural environment [1,2]. This leads to the threat to all aquatic life organisms and affects our health in humans and animals even at low concentration of toxic heavy metal ions. For instance, lead is considered as highly toxic, which causes damage to kidneys and cadmium associated ions, leading to bone mineral loss. The primary pollutants from wastewater seen as textile dye, plastics, mining, and electroplating from industries with the combination of different heavy metals reach nearby areas of rivers.
Heavy metals are naturally occurring elements, which have high density and atomic weight. These are five times stronger than the water in terms of density. Due to this toxicity in polluted waters, when people drink this water, it affects them, with respect to age, gender and genetics. The consumption of this water, also leads to carcinogenic counterparts in the body and leads to death along with the shutdown of organs due to their high level of toxic concentrations. The heavy metals are difficult to degrade in the natural environment [3]. To treat wastewater, there are two kinds of methods that can be followed. The physicochemical methods cost high and they are used to treat toxic impurities ions, but these are considered as incomplete methods in the removal of heavy metal ions [4]. Thus, this is a limiting factor in the development of strategies for treating heavy metal pollution.
Due to this, another alternative method that has emerged is algae bioremediation. This is an environmentally safe, economical and reliable process for removing metal ions from wastewater But even in this method, it is difficult to cultivate algae biomass, because of the selectivity of different strains of algae and its ability to absorb, on its own level of capacity. So, detailed characterization of algal culture is required [5]. Dumping of waste water into lakes, ponds creates toxic and bad odor. This waste water travels to nearby villages or cities where the usage of water from lakes and ponds is found to be 8 % for domestic and 70 % for agriculture. In other words, environmental pollution results due to the amount of heavy metal and chemical textile effluents released [6].
The term algae can be defined as simple non-flowering plants mostly found in aquatic zones. They have chlorophyll but lack roots, leaves, stems and vascular tissue. In other words, algae can also be called seaweeds. Algae that are found in oceans, lakes and rivers produce half of the oxygen on the planet which is beneficial for all living things in terms of breathing. These algae were formed during the Paleozoic era which is over 480 million years ago and started to give life and grow as plants to produce fern and other bryophyte organisms. In algae, there are different ranges of species, among these brown algal species are more efficient when coming to terms of biosorption of heavy metals and its capacity of sorption. But before focusing on brown algae and studying heavy metal interaction, it is important to understand various groups of colors of algae [7].
The sorption capacity of an algae cell surface, to a particular ion depends on the number of functional groups present in the algae cells. The functional groups of the cell surface influence the coordination number of the metal ion to be absorbed, binding groups of the metal ions, and complex formation of metal ion [8,9]. Every binding group makes the net charge on the cell surface as negative one. Algae cell wall forms the first barrier against heavy metal ions and proteins, polysaccharides creating the binding sites. Algae strains that have different kinds of cell wall and its capacity of metal ions bio sorption will vary. When it comes to the color of algae strains, there are three different color ranges. In chlorophylls, green algae are seen and Carotenoids involve the color of red, orange, yellow, amber, or brown. Among this brown having more ion affinity and considered as a good strain. Lastly, Phycobilins have red or blue color algae strains. In algae, some factors affect the heavy metal ions during bio-sorption such as pH, temperature, presence of competing ions, metal ions and algae biomass concentration [10]. The different types of algae are shown in Figure.1.
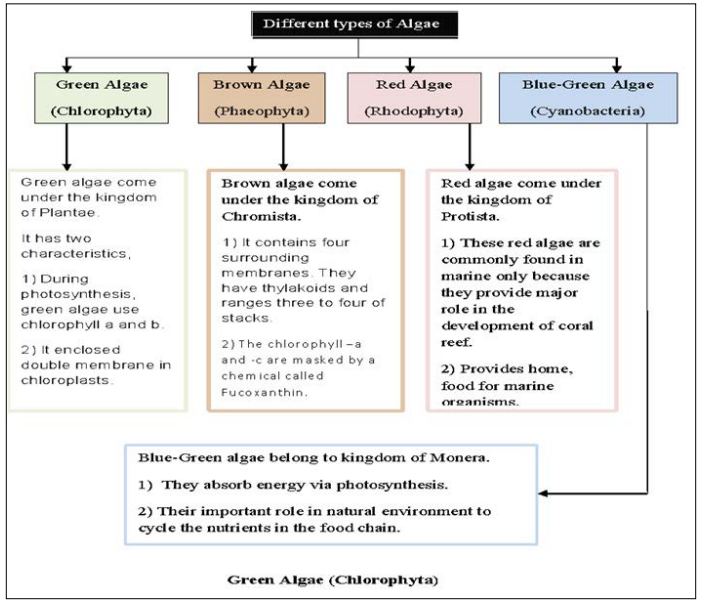
Figure 1: Types of Algae algae. In comparison with green algae, red algal marine species
These are estimated to be around 5,000 to 7,000 algal species. In these, 90% of the algae species are considered as freshwater and remaining are marine organisms. The green algal species conquered the land due to its rapid growth and most importantly they have their origin dated to the Paleozoic era. Hence, these green algae species can be considered as the ancestors for most of the plants living today and from the category of colored algae [11].
The brown algae look smaller but has stronger rigid surface than any other algal species due to active rich minerals. These are estimated to be around 1,400 to 2,500 species. Almost all species of the brown algae are found in marine only, but in rare cases also able to form in rivers in fewer amounts. In brown algae, there are various groups such as giant kelps that are called Macrocycstis and Sargassam groups. These sargassam species have pharmaceutical components which are similar to red algae but these are better than red algae because of its sturdy nature [12].
It is estimated that there are around 3,000–4,000 species of red is about 90% in its population. Some unicellular red algae are found in sea shores. Some red algae make calcium carbonate structures and coralline to support life balance on the rock of the shores and provide nutrients for algae to other marine species such as fish. These red algae are unique because of having various pharmaceutical components, which are used in antimicrobial applications [13].
The Blue-Green algae are abundant and they have nickname as hardworking photo synthesizer. They are present in the lakes, ocean. These don’t have any pharmaceutical components but are able to withstand hostile environments such as snow, thermal hot springs, deserts which is a unique ability in these species. They are estimated to be around 3000 to 6000 species. These blue–greens algae can achieve more algal bloom in lakes and they appear as red, when more density of algal bloom is formed [14].
The significance of using algae biomass is due to the sustainability in its process and low cost in industrial scale bioremediation.
Sometimes, this waste water serves as an enriched source to cultivate algal strains with better bio-sorption capacity for the removal of metal ions. The term “bio-sorption” refers to accumulation of heavy metals from toxic water that cleans by physicochemical uptake or metabolically mediated. In other words, “bioaccumulation” describes the process of removal of metals during the metabolic activity of an organism [15,16].
In recent years, studying mechanisms of bio-sorption on algal biomass shows that, for either, in separation of metal from industrial effluents or to recover metal from the solution in waste water, brown algal biomass is highly effective with metal during interaction on cell walls. The probable reason is due to the presence of rich nutrients of polysaccharides [17]. This alga innovative technology makes bio-sorption as an efficient way to remove heavy metal ions from wastewater. In some cases, the heavy metal toxic ions make algae cells poisoned which leads to cell death. But apart from that, the sorption process shows larger variations of growth of algae. There are two kinds of algae such as living algae and non-living algae [18]. Various environmental factors affect the bio-sorption capacity of the living algae. This is found to be more complicated in living algae rather than non-living algae, as it takes place in the growth phase and the intracellular uptake. The absorption of metal ions takes place on the surface of the cell membranes of the non-living algae termed as extracellular process. They are treated as a group of polymers that contain sugar, pectin, cellulose, etc. In heavy metal accumulation there are two kinds of phases [19].
Heavy metal ion removal involves large initial concentration of metal ions and observing the interaction of algae cells and amount of toxic concentration produced such things will help us to develop solutions for treating waste water. During metal-algae interaction, structural and binding proteins form metallothionein (MT) which bind to the adsorbed ions, avoiding accumulation of heavy metal ions into the host cells of algae. The M denotes metal where they bind to the cell wall and during binding, the accumulation of metal present over the cell wall is transported into the cytoplasm [20]. Not only metal ions but also functional groups such as Hydrogen and other molecules such as polysaccharides, extracellular polymers get absorbed during interaction. This is shown in Figure: 2.
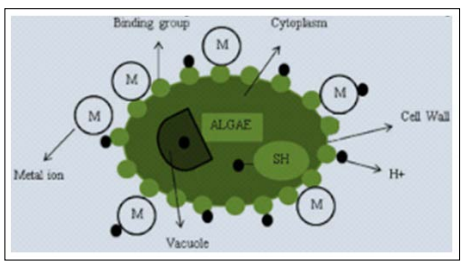
Figure 2: Algae cells interacting with metal ions over the cell wall (SH is a binding group which is a metal ion
The amount of toxicity produced in metal ions leads to formation of protein structure denaturation and the replacement of essential features in living algae is important and another most important factor is that its functional groups where it contains sulfonic acid of fucoidan. Sulfonic acid which plays a secondary role and the hydroxyl groups that present over in the cell wall consists of fewer amounts of polysaccharides. Due to this it becomes negatively charged and pH which is lesser than 10, this also plays a secondary role in pH [21]. Among the species of brown algae, Laminariales and Fucales, which is the division of Phaeophyta, are considered as important groups. The field of bio-sorption during interaction between algal biomass and metals shows rich bio-sorption activity in the cell wall where they have a greater number of extracellular polymers and polysaccharides. These brown algae are highly complex in nature and have the ability to withstand a wide range of heavy metals and toxic concentrations in higher amounts, when compared to other diverse species of algae. Fucales have more nutrient rich cell wall and bio-sorption capacity, while the other species Laminariales have the same capacity but these are less in comparison with Fucales when bio-sorption is concerned. These two species are in the leading position in comparison with other colored algae or brown range of species and also these brown species present flagella [22].
In cellular structure, the chloroplast envelope contains chloroplasts which have three thylakoids (appearance as disc shaped) that present per band. They contain another structure called plastid, which is important for chlorophyll as well as source of energy. During depletion of energy in algae, the stored food material will be transported to algae and retains its energy into its previous state. In chlorophyll, they have three chloroplast envelopes such as a, c1, c2. In addition to its chloroplast envelope, these are surrounded by another two membranes of the endoplasmic reticulum which is also known as Cer [23]. Fucale, the brown algal species, consists of a nuclear envelope which is present as outer membrane which encloses an inner membrane which is found to be discontinuous but, in the species, Laminaria, it is found to be continuous. All algal species are coded for nuclear DNA but in some circumstances few proteins are coded from within the chloroplast [24].
This interaction between algae and metal ions plays important role and depends on various factors such as
PH is considered as a crucial parameter, when uptake of metal ions by the algae biomass is concerned. This uptake results in different functional groups and changes in its behavior in the surface of algae cells. This allows complex formation. Non-appearance of H+ ions or oxidation tends to increase the ability of metal cations-ligands. Reduction of bio-sorption capacity will happen when functional groups present in acidic solutions are transferred which prevents binding cations to the functional groups. It is important to find optimal PH for algae that makes strong surface charge, absorbing sites and degree of ionization. In bio-sorption, the first step is to diffuse ions in the algae cell surface which is negatively charged. The metal ions are absorbed by algae cells creating depleting growth media of metal ions and increase in algae growth. Therefore, bio-sorption has a major role when metal ions are in the dilute media [25].
When the amount of algae biomass increases, the concentration of metal ion uptake reduces. In other words, increasing its biomass concentration will not only remove heavy metal ions, but also affect bio-sorption capacity negatively. During electrostatic interaction, algae cells which have effect on metal ions will uptake biomass concentration, this is also known as “shell effect” and the outer structures, in which the functional groups bonded to metal ions will be avoided. Using different algal strains and metal ions, variable mass in bio-concentration can be created [26].
The temperature is one of the most important affecting factors of algae for survival and each algae species has a different response to temperature. The interaction between metal-ligand complexes also has temperature changes in its function. Some studies claim that increasing the temperature of algal culture leads to increase in metal biosorption capacity. These may be due to,
Other studies claim that some algae go exothermic during metal ion uptake, and hence as this uptake is increased in its capacity and temperature decreases. During intracellular absorption, enzymes that participate in the transfer of ions in living algae, leads to increase in temperature that creates greater impact on absorption capacity [27].
The contact time of the heavy metal ions are highly taken into consideration because of interactions on bio-sorption and cell surface, and it occurs in two stages,
Heavy metal ions are passive and it occurs rapidly in non-living algae cells. But in living algae, there will be a greater bio-sorption capacity [29].
In a multi metal ion system, concentration and bringing different heavy metal ions varies. For example, a mixture of metal ions that contains nickel, cadmium, copper and these together can be called multi metal ions. It becomes necessary to remove metal ions together, instead of single ions, which is time consuming [30]. To remove multi-metal ions, they can be grown in algal growth media and there is a possibility that the effect of cadmium removes nickel and so on. This creates simultaneous bio-sorption on algae cells. Other factors are that during uptake of every metal ion in wastewater, the algal cell sometimes cannot sustain because the cells may get poisoned and moreover changes in its abnormal growth leads to cell death. But this can be treated with pretreatment such as calcium chloride and other immobilization techniques. These multi metal ions sweep entire metal ions that fill in ponds and lakes, which makes the process of cleaning toxic wastes easier. Thus, the adsorption process of multi metal ions shows a significant approach than that in single ion solution.
The uptake of heavy metal ions by algal biomass can be increased by physicochemical methods but they cause secondary pollution. So, physical treatment methods can be used, such as freezing, boiling, crushing and drying which may lead to increase in its bio- sorption capacity and expand the level of capacity while treating a wide range of heavy metals. Some other pretreatments involve calcium chloride, formaldehyde, sodium hydroxide, hydrogen chloride and gluteraldehyde [31].
Algae species range from fresh to salt water and when biosorption takes place, marine brown algae are considered as the best active process in absorption. Some of the uptakes of metal by brown colored algae are shown in Table.1.
|
Algae species |
Heavy metal treatment |
|
Ascophyllum nosdosum |
Lead, Zinc |
|
Fucus Vesiculosus |
Nickel, Copper,Cadmium, Chromium |
|
Laminaria japonica |
Same heavy toxic metals (above) |
These different pretreatments on metal show varied bio-sorption capacity and in some cases, weaker cell walls in algae cannot sustain when high amounts of toxicity enters into cytoplasm. But these are solved with pretreatments and making improved versions of algal biomass with high active biosorption. Also, these algae species come under the genus of Sargassum. This is sorted out in Table.1 The accumulation of metal ions present in both living and nonliving grown algae shows an ion exchange process whereas living algae depends on the accumulation of metal ions while non-living algae are dependent on the bio-sorption of metal ions. But apart from that, some parameters such as pH, temperature and contact time affect the process during biosorption of heavy metal ions; Non-living algae have more advantages in bio-sorption than living algae. Non-living algae can be used to recycle dead biomass by nourishing them [36]. For example, metal ions bind with algal cells that can be removed by desorption process (calcium chloride, hydrogen chloride, calcium chloride) and deionized water. But living algae do not possess mechanical resistance [37]. For solutions, with heavy metal ions and pH that favors treatment with immobilization techniques such as flocculation, cross-linking, covalent binding to carriers etc [38].
These supporting materials of biopolymers (Alginate) or synthetic compounds (silica gel) can be used for immobilization of biomass. Among these, synthetic compounds of polyacrylamides are mostly used because it is more resistant than calcium chloride, but one disadvantage is that these are high cost and creates toxicity in living algae cells. Thus, algae immobilization has huge potential for removing toxic wastes in heavy metal pollution [39].
The brown algae are a significantly efficient class of bio sorbents in comparison with other algal species [40]. Luckily, they are cheaper, have good metal–selectivity, better metal ion uptake makes it, suitable solution for treating algae in wastewater bioremediation which can be employed in many useful applications. The implementation of algae bio-sorption technology in industrial and environmental remediation also needs better understanding about its parameters, initial concentrations, contact times and physicochemical method conditions.
Successful bio-sorption process involves increasing the biomass productivity of wastewater full grown algae; it is also attractive when combining them with waste water treatment with downstream processing for cultivation. In brown algae, the order Laminariales and Fucales under the division of Phaeophyta shows important aspects when coming to the field of biosorption. The biosorption capacity is unique because it binds faster, able to accumulate various metals in one swoop instead of cleaning single metal and also, its pretreatment changes the behavior and structure and helps improve further in its capacity for absorption [31]. Also, the concepts used in biochemicals are used in equilibrium state and the process of heavy metal that binds with algal cells becomes more realistic than ever [41,42].
The important advances that emerged in recent years are showing significant improvement when compared with the goals in earlier days. Thus, algae became a good revolutionary technology for cultivating abundant algal biomass and treating them with industrial waste water.
