Author(s): <p>Temiloluwa Ojuolape Amusan*, Jonathan Maduka Nwaedozie and Femi Emmanuel Awe</p>
Background and Objective: Fire retardants are substances that are applied to the surface of any combustible base which can delay the spread of fire or keep the structure intact against fire, thereby allowing sufficient time for safety measures to be taken. In this study, the production and chemical characterization of fire-retardant paint was achieved. Materials and Methods: The components used in the production of the paint include: Ammonium dihydrogen phosphate, Melamine, Urea, Gum Arabic, and paint base. The coating was prepared by blending all the components. Agilent Technology Cary 630 Fourier Transform Infra-Red (FTIR) analyzer was used to determine the structural characterization of the fire-retardant paint. A Horizontal burning test following the ASTMD635-75 standard procedure was employed to evaluate the flammability properties of the coated samples. The pH of the paint was determined with the aid of a pH meter. Density and percentage weight gain of the coated materials were also carried out. Results: The results obtained showed the density of the paint to have values ranging from 1.046 g/cm3 to 1.066 g/cm3 while the pH values range from 5.05 to 4.90. The FTIR spectra confirmed the presence of -NH2 amide group, C=O carbonyl group, C=C conjugated structure, C-O Stretching of inorganic carbonate, CH Aliphatic group, and C-O of carbohydrate. The Flammability measurement showed the burning rate to range from 2.227 mm/s to 1.25 mm/s. The ignition times were found to range from 3 to 4 s. The flame spread test values range from 6 to 8 s. Conclusion: From the results of the burning analyses, level 4 had the highest flame-retardant properties. There is a clear difference in the ignition time and burning rate of the coated samples compared to the uncoated reference.
Fire retardants are substances that are applied to the surface of any combustible base such as wood, bricks, and metals to form a protective and decorative fireproof coating [1]. Cellulosic base composites like plywood, particle boards, and fiberboard are widely used in building constructions [2]. These materials are highly combustible and often pose serious fire hazards [3]. Nowadays, the rapid changes in building and development in architecture and technology have led to more complex structures with a wide range of materials in the form of organic or inorganic and synthetic or natural materials used in buildings being easily flammable or readily burns on exposure to high temperature of fire [4]. On the other hand, non-combustible materials such as metals and concrete will neither burn nor support combustion or release flammable vapors when subjected to fire. However, they can withstand fire for a certain period, making their strength deteriorate significantly at high temperatures. The devastating nature of fire creates havoc that results in great loss of both lives and properties. It can also cause great human suffering and financial losses. If these materials are suitably and properly treated for fire retardancy, it will decrease the growth period of fire and reduce the spread of flame thereby preventing the occurrence of fire hazards and loss of life and properties. Traditionally, fire retardant coatings typically make use of compounds that contain halogenated compounds like chlorine and bromine [5]. The coatings formulated from these compounds are more effective in the gas phase and act in the flame zone by forming a blanket of halogen vapor that interferes with the propagation of flame that interrupts the generation of highly reactive free radicals, thus helping in extinguishing flame. However, the release of toxic and corrosive gases generated by these halogenated compounds while burning is ecologically unsafe, posing a lot of potential risks to human health and the environment [6,7]. Recently fire retardants based on Nitrogen compounds are found to be of interest to researchers due to their environmentally friendly nature. Nitrogen-based fire retardants have the following advantages, these include low toxicity, absence of dioxin, and halogen acids, and low evolution of smoke unlike halogen-based flame retardants, they do not interfere with the set of stabilizers added to the material [8,9]. Also, fire retardants based on Nitrogen compounds are suitable for recycling due to their high decomposition temperature. Moreover, they are environmentally friendly because they do not add any new elements to those already present in the polymer composition. Given the foregoing, this research work will focus on the production of nitrogen-based flame retardants synthesized from agricultural materials.
Research on the production of fire-retardant paints is very scarce; therefore, the objective of this research is to determine the ignition time, flame spread time, burning rate, and percentage weight loss of fire-retardant paint produced from ammonium dihydrogen phosphate, melamine, and urea.
This research was conducted in the chemistry laboratory Nigerian Defence Academy, Igabi Local Government Area Kaduna, Nigeria. It is located on latitude 10 0 609’ and longitude 70 429’ North of the Greenwich meridian. The research was conducted within a period of seven months; From February 2022 - September 2022.
All the raw materials that were used for the production of the fire-retardant paint were locally sourced. 2 kg Raw gum Arabic was purchased from Kawo market, Kaduna. The wood that was used as substrate and urea was purchase from Mandom mando. Ammonium dihydrogen phosphate was purchased from Nec. Nigeria Limited, Kano Road Kasua, Kaduna. While all other raw materials were purchased from Sunny Wax Paint, tundunwada, and Kaduna.
The industrial-grade Chemicals that were used for the production of the fire retardants coating include the following: melamine, urea, titanium dioxide, polyvinyl acetate, Calgon, nitosol, genepour, gum arabic, calcium carbonate, formalin and Ammonium dihydrogen phosphate (analytical grade).
The equipment that was used in the course of the research included: HI8424 General purpose pH/mV Meter, Note Book series Digital scale, Binatone electric mixer; son: MPR/EEE/0042. stopwatch, methane source bunsen burner, and Agilent technology 380 FTIR analyzer.
The preparation of the fire-retardant coating was carried out by blending all the components together[10] The coating was prepared as follows:
A 500 cm 3 of water at room temperature was measured into an electric mixer; 7.3 g of Calgon was added and mixed for two seconds, then 11.20 g of titanium dioxide was added and mixed (for 1 second), 16.90 g of calcium carbonate was added and mixed, then, 23.2 g of polyvinyl acetate, 7.6 g of urea, 1.1 g of nitrosol and 1.6 cm3 of genepour with 0.7 cm 3 of formalin were separately added and mixed for 2s. Then 22.80 g of ammonium phosphate was added and mixed for 1s, and 7.60 g of melamine was added. The mixture was mixed thoroughly for 5 seconds and weighed. The paint produced was transferred into a container and covered tightly.
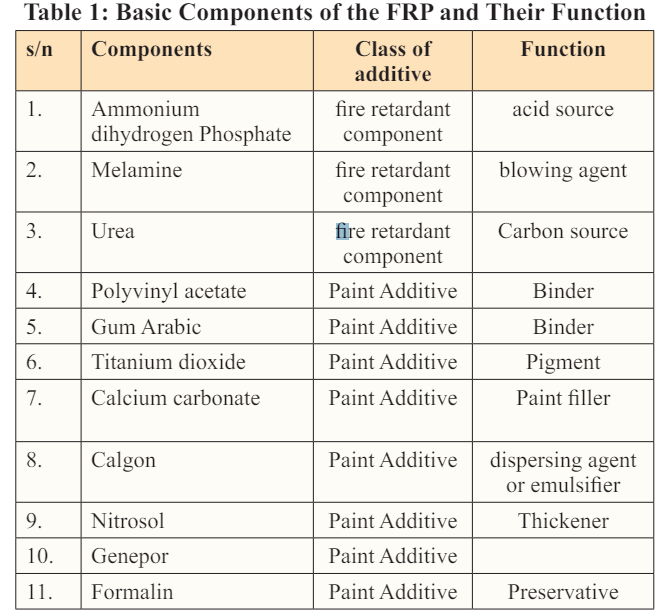
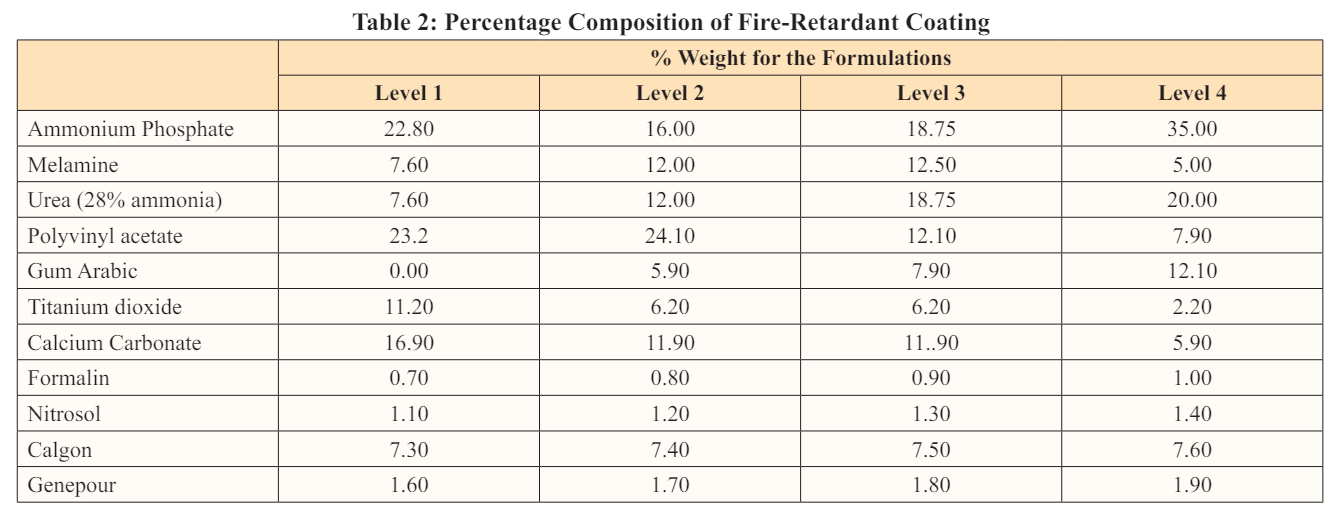
The same procedure was followed for levels 2, 3, and 4. The basic component of the -fire-retardant paint was presented in Table 1 and the percentage composition of each component was presented in Table 2. The pictures of the fire-retardant paint produced were presented on plates 1 - 2
The following are the basic components for the production of fire-retardant paint and their respective roles:
1. Fire-Retardant Additives: include: Ammonium dihydrogen Phosphate which acts as the acid source. ii. Melamine and Urea: which acts as the blowing agent. iii. Gum Arabic: which acts as the carbon source.
2. Binders: These include an inorganic resin, Polyvinyl acetate, and an organic polysaccharide compound, Gum Arabic.
3. Paint Additives: These include: Titanium dioxide (pigment), Calcium carbonate (paint base or filler), Calgon (dispersing agent or emulsifier), Nitrosol (thickener), Genepor: and Formalin (preservative) (Table 1).

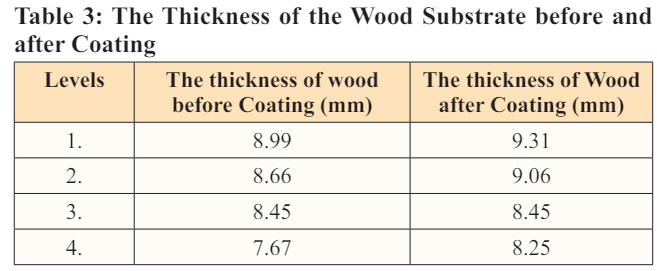
The wood (cellulose substrate) that was used for this research was first polished into a smooth and uniform surface and then cut into 15 cm x 10 mm long as shown in plates 3 and 4. The thickness of the wood before coating was 8.99 mm, 8.66 mm, 8.45 mm, and 7.67 mm, and after coating was measured to be: 9.31 mm, 9.06 mm, 8.45 mm, 8.25 mm (Table 3).

The pH of the paint samples was measured with the aid of a precalibrated pH meter. All the measurements were carried out at room temperature.

A horizontal burning test was carried out to evaluate the burning rate and extent of the burning of the coated samples tested in a horizontal position according to American Standard for Testing of Material, ASTMD 635-75 standard [11]. The dimensions of the samples were 15 x 10 x 6 mm. Edge exposure was carried out when the flame was applied to the bottom edge of the test sample. The time taken to ignite the tiniest particle of the wood was taken as the ignition time. The burning rate and percentage weight loss of the coated samples were determined.
The active functional groups present in the paint were assessed by Fourier transform infrared (FTIR) analysis using Agilent Technology Cary 630 instrument at room temperature. The samples were scanned over a spectra range of 4000- 650 cm-1 with a resolution of 8 cm-1.
The data obtained were analyzed using Microsoft Excel and IBM SPSS (version 23). The dependent parameters were varied at four levels with each having three replicates. The average values were determined and applied as the representative data.
The pH values of the paint samples produced fall into the range of 5.05, 5.03, and 5.24 for levels 1, 2, and 3 respectively, while level 4 has a pH of 4.90 as shown in Fig.1. This implied that fireretardant paint is slightly acidic due to the composition of the fire-retardant paint. Research reported that samples that contain gum Arabic solution in their compositions had lower pH values as compared to samples that do not have [12]. This shows gum Arabic also can lower the pH of paint.
It was reported in the literature that the standard value in paint production according to SON (Standard Organization of Nigeria) was a pH above 9.0 [13]. Fig.1 showed the bar chart representation of the pH of the paint.
Ammonium dihydrogen phosphate is also responsible for the reduced pH of the Fire-retardant paint. Research showed that Physicochemical properties like stability and shelf life of the paint (which is a direct function of its pH) were affected by the components used in manufacturing the paint [14]. From the result obtained, the pH of the paint decreases as the percentage of ADP increases. Level 4 which has the highest amount of Ammonium Dihydrogen phosphate has the lowest pH of 4.9. The graphical representation of pH against ammonium phosphate is shown in Fig. 2.


From the result obtained, level 2 has the highest percentage of weight gain. Due to the highest percentage of binders (polyvinyl acetate) in its composition. This showed that level 2 adheres to the substrate better and has better applicability on the wood. Table 4 shows the percentage weight gain of the coated samples.
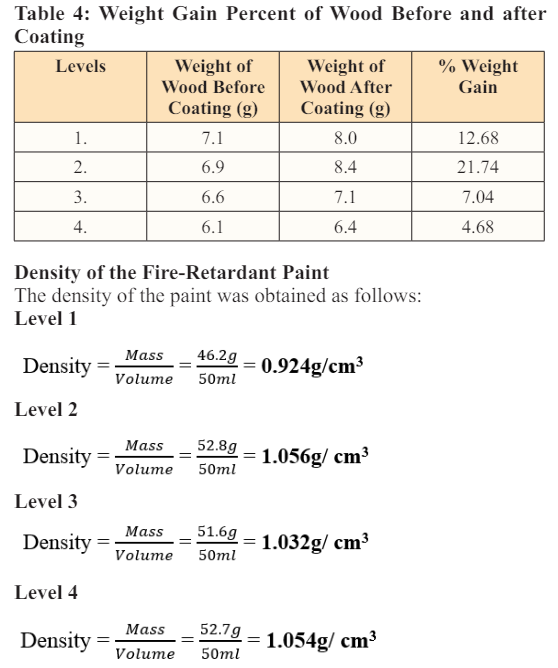
The density of the paint samples produced fell within the specified range by ENISO 2811-1 Standard. From the result obtained, level1, has a density of 0.924g/cm 3 , while levels 2, 3, and 4 have higher densities of 1.056 g/cm3 , 1.032 g/cm3 , and 1.054 g/cm3 respectively (Table 4) due to the presence of gum Arabic in the component of the paint. Level 1 which has the lowest density has 0% gum Arabic. This showed that gum Arabic has a contributing factor in increasing the density of the paint. Abdusalam and Maiwada [15]. Fig. 3 shows the bar chart representation of the density of the paint.
Gum Arabic has a pH range between 4.5 to 5.0 [16]. The Density and brightness of the paint change as the percentage of gum Arabic increases. Level 1 which has 0% gum Arabic has the lowest density of 0.49 g/cm3 and the brightest color while levels 2,3, and 4 have an increased density and dull color. This was similar to what Abdusalam and Maiwada [15] obtained in their result.
Table 5 shows the ignition time, flame spreading time, burning rate, the extent of burning, and % weight loss of the coated wood samples. The ignition time for control and Level 1, 2, 3, and 4 were 2 s, 3 s, 3 s, 4 s, and 4 s and the burning rate for control and Levels 1, 2, 3, and 4 were 0.033 g/s, 0.015 g/s, 0.016 g/s, 0.008 g/s, and 0.009 g/s respectively. There is an average decrease of 63.6% in the burning rate of the uncoated samples compared to the coated sample. Ofuyatan, O. et., [17], reported a decrease of 48.9% in the burning rate of a sample coated with fire retardant paint [17]. Research showed that the Flame Propagation Rate and After Glow Time of both emulsion and gloss coated wood splints decreased with increase in concentration of the fire retardant. On the other hand, the ignition time was increased [18,19].

From the result of the analysis, the flame spread between 3-4s after ignition and the total burning time was 6s.7s, 8s, and 8s for levels 1, 2, 3, and 4 respectively. This implies that when the coatings have burnt for these times only 0.09 g, 0.11 g, 0.06 g, and 0.07 g of the substrate got burnt at a very slow rate. This complied with the mechanism of an ideal fire-retardant coating as reported by Marriapan 4 in his report. The purpose of the fire-retardant paint is to stop flame propagation and prevent the substrate from being consumed. The burning rate of the coated samples slowed down compared to the control. Level 4 has the lowest burning rate. Figs. 4 and 5 showed the graphical representation of the ignition time and burning rate of the -fire-retardant coating.

Effect of Ammonium Phosphate, Melamine, Gum Arabic, And Urea on The Flammability Properties of The Fire -Retardant Paint When the active components of the fire-retardant paint i.e. ammonium dihydrogen phosphate (a catalyst), melamine (blowing agent), Gum Arabic, and Urea (carbonaceous materials) are exposed to fire, the ammonium phosphate decomposes to produce phosphoric acid which acts as a dehydrating agent. the polysaccharide salt (Gum Arabic) upon hydrolysis with the help of melamine and urea is dehydrated by the acid-forming a large amount of carbonaceous material that form a noncombustible barrier to protect the wood substrate from burning. As a result, flame propagation is stopped. Thus, making the flame spread time and the burning rate of the coated substrate slow down. Weil20, reported the interaction of melamine with phosphorus acid by the decomposition of ammonium phosphate which leads to the generation of ammonia gas and the formation of melamine phosphate salts.
The combustion reaction of ammonium phosphate which takes place within the paint on heating is given in equation 4.3:
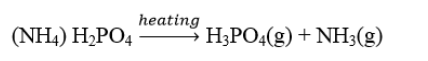
The chemical structure of the FRP components was confirmed by FTIR. There is a broad Peak at 3265 cm-1, 3257 cm-1, and 3272 cm-1 which confirms the presence of an amide -NH2 group from Melamine and Urea. The Characteristic peak around 2119 cm-1 (level 1), 2112 cm-1 (level 2), 2109 cm-1 (level 3), and 2117 cm-1 (level 4) is attributed to the C=C of a conjugated structure coming from the presence of polyvinyl acetate. Also, there is a sharp peak around 1636cm-1 (levels 1,2,3, and 4), which is attributed to C=O of a carbonyl group from urea and polyvinyl acetate. The peak at 1457 cm-1 (level 1), 14553 cm-1 (level 2), and 1461 cm-1(levels 3 and 4) is attributed to C-O stretching of an inorganic carbonate coming from calcium carbonate. The disappearing peak around 1364 cm-1 (Level 1and 1371 cm-1 (level 2) is attributed to CH bending of an aliphatic group which is coming from the presence of gum Arabic and polyvinyl acetate. Also, the strong peak at 1069 cm-1 (Levels 1 and 4), and 1073 cm-1 (level 3) is attributed to the C-O of a carbohydrate which is also coming from Gum Arabic. Summarily, the result of the FTIR showed that the existence of all these bonds and functional groups indicates the presence of all the active ingredients necessary for the flame retardancy of the paint produced.
Given future research, the following recommendations were made:
i. It is recommended that a gloss-type fire retardant paint should be employed for wood and cellulose substrate for the paint to have better adhesion with high water resistance.
ii. To prolong the shelf life of the paint and for better stability, the pH of the paint needs to be adjusted to above 9.0 by increasing the percentage of CaCO3 or by adding soda ash and more preservatives.
iii. To increase the ignition time and lower the burning rate of the paint, the percentage of ammonium phosphate should be increased and it must be ensured that all the chemicals are still potent and within their shelf life.
It is concluded that the gum Arabic can be used in conjunction with polyvinyl acetate as binder in the production of fire-retardant paint. This would lower the cost of production since the cost of gum Arabic is lower than that of polyvinyl acetate. Also, ammonium dihydrogen phosphate, melamine, gum Arabic, and urea are effective in offering fire retardant properties in the production of emulsion-type fire retardant paint. The density of the paint samples produced fell within the specified range by ENISO 2811- 1 Standard. The pH of the paint is lower than that of the ENISO standard as a result of the composition of the paint, this could make the paint to be susceptible to bacterial growth after a long time.
Amusan T.O. executed the research; Prof Nwaedozie J.M. supervised the research; Dr. Awe F.E. coordinated the research activities.
The authors declare no conflict of interest
