Author(s): Nidal Abdelgadir Elbala Elmagboul
Aim: assessing the prevalence of diabetic peripheral neuropathy and its associated risk factors in diabetic patients attending primary health care centers in Khartoum locality, Sudan 2019.
Materials and Method: Descriptive cross-sectional facility-based study, 288 patients were assessed using a questionnaire consist of two-part: the first part is regarding the sociodemographic data and the second part is a standardized MNSI questionnaire.
Results: out of 288 patients 223 were females, the mean age was 53.2 (SD ± 9.9 years), the majority had type 2 DM (98.6%). The overall prevalence of DPN was 42%, males were 31.4% and females were 68.4%. The risk factors for peripheral neuropathy were the female gender, the age of the patient more than 58 years and the duration of diabetes more than 10 years, smoking, being married and divorced, being overweight, being a householder, using oral hypoglycaemic agents, and being on poor glycaemic control with a p-value of 0.002, 0.001, 0.001, 0.012, 0.001, 0.001, 0.001, 0.024 and 0.001 respectively. In the logistic regression model the bad glycaemic control and the marital status (being married) emerged as significant independent predictors for developing DPN.
No significant association was found with the educational level of the patients (p-value = 0.127) and type of diabetes mellitus (p-value = 0.178).
Conclusion: high prevalence of peripheral neuropathy in diabetic patients attending primary health care center. Higher age, longer duration of diabetes, using oral hypoglycaemic agents, and poor glycaemic control were important risk factors for it
Peripheral neuropathy is one of the most common complications of both type 1 and type 2 diabetes and the greatest source of morbidity and mortality in diabetes patients. Peripheral neuropathy (or diabetic polyneuropathy) can present as a loss of sensation that can lead to neuropathic ulcers, and it is a leading cause of amputation [1,2]. It is the most common type of neuropathy in people with diabetes [3,4]. Peripheral neuropathy may be asymptomatic. When symptoms are present, they may be negative or positive. Negative symptoms include loss of sensation and loss of strength, while positive symptoms include pricking or pain [4]. One of the most distressing symptoms that people can suffer from is a neuropathic pain and paraesthesia and this can severely impair the quality of life [1].
In a population-based study [5], 22% of the diabetic cohort had peripheral neuropathy that was graded as either moderate or severe. Long-standing peripheral neuropathic pain associated with peripheral neuropathy occurs in one of six diabetic subjects [6]. It is estimated that the prevalence of peripheral polyneuropathy in diabetes patients is approximately 25-50% in developing countries [7,8]. A DPN account for more hospital admissions than all other diabetic complications combined and is responsible for 50 - 75% of non-traumatic amputations [8,9]. Painful DPN is associated with a high degree of functional impairment, impairment in health-related quality of life, and activities of daily living [10,11]. Painful DPN reportedly results in significantly higher healthcare costs when compared with age and sex-matched diabetic patients without DPN [12,13]. Considering the priority of the problem and its increasing co-morbid complication, there is an undeniable need to prepare primary data for more awareness of stakeholders and better policy recommendations [14]. To address this issue, we should provide comprehensive scientific evidence that supports policy actions, program monitoring, and intervention evaluation [15]. This study aimed to assess the prevalence of DPN in diabetic centers in the Khartoum state.
Peripheral neuropathy is the most common risk factor for foot ulcers in diabetic patients contributing to higher than 80% ofthese ulcers [16,17]. Although diabetic foot is a quite common complication among diabetics, it is frequently ignored [18], a condition which is not only associated with high costs of treatment and care due to its prolong the length of hospitalization stay and increase risk of amputation but also places a heavy burden on the society [19]. Reports have revealed that neuropathy, diabetic foot, and amputation account for some 18% of the overall burden - calculated using Disability Adjusted Life Years (DALYs)- placed on Iran in 2001 [20]. The prevalence rate of diabetic foot in the world is about 3% [20]. Accordingly, statistics show that a diabetic somewhere in the world loses his/her leg every thirty seconds [21]. The foot ulceration is not only the most common complication of neuropathy but also among the preventable diabetes complications [19]. Identifying at-risk patients, hence, can preclude the development of a large number of foot ulcerations. The objectives of this study is to estimate the prevalence of diabetic peripheral neuropathy, to assess the risk factor of diabetic peripheral neuropathy and to assess the association between diabetic peripheral neuropathy and the risk factors that will help to provide primary data for more awareness of stakeholders and better policy recommendations.
This study was a multicenter, cross-sectional study conducted in PHC centers in Khartoum locality, Khartoum state, Sudan. The three PHC centers were selected randomly and two hundred eighty-eight diabetic patients were involved. Eligible were adults over 18 years who were diagnosed as type 1 or 2 diabetes mellitus. We exclude individuals with other neurological or pain conditions not associated with DPN. Ethical approval had been taken from the Department of Community Medicine, University of Khartoum, Sudan. Permission had been taken from the federal ministry of health, Sudan. Approval had been taken from Michigan university to use the MNSI questionnaire.
After taking written informed consent an interview-based
questionnaire was administered to each of the participants in a
homogenous manner to limit interviewer biases. The questionnaire
was consisting of two-part: The first part is regarding the
sociodemographic data of the patients including the age, gender,
marital status, occupation, residence, education, type of diabetes,
duration of diabetes, and type of treatment using and measuring
height and weight to calculate BMI and measuring the HbA1C.
The second part is the MNSI questionnaire which is a structured
questionnaire compose of two-part the first part is to assess
the symptoms of PN of the participants and a participant who
answered positively will shift to the second part which is the
clinical examination; examination of the feet appearance for dry
skin, calluses, fissures or ulcers, infection and the evaluation of
the fine touch using 10 g monofilaments vibration sensation be
the tuning fork and ankle reflex by hummer. A diagnosis of DPN
established be prior validation studies is >2 out of a total score
of 8 in the MNSI questionnaire.
Data was Analysed partly manually and mostly by using SPSS
software (version 21), it had been entered and encoded by the
researcher and then frequencies of the sociodemographic variables
had been calculated, the prevalence of peripheral neuropathy
had been calculated and the association had been tested using
T-test, chi-square test, and logistic regression. Final results were
displayed and presented in the form of tables and graphs.
Out of 288 patients, 223 was females (77.4%) and 65 males (22.6%) [figure 1], the mean age was 53.2 (SD ± 9.9 years), the minimum age was 23 and the maximum was 73, the majority had type 2 DM (98.6%). Age distribution was found as follows: under 40 years were 24 represents 8.3%, 41-60 years were 176 represents 61.1% and 61, and above were 81 represents 28.1% [figure 2]. Regarding occupational background, housewives were 184 (63.9%), free workers were 22 (7.6%), employees were 41 (14.2%), retired were 12 (4.2%) and non-employed were 29(10.1%) [figure 3]. Regarding marital status singles were 6 represent 2.1%, married were 256 represent 88.9%, divorce was 3 represent 1% and widow was 23 represent 8% [figure 5]. Regarding educational levels primary school was 16 represents 5.6%, the secondary school was 144 represents 50% and higher education was 128 represents 44.4% [figure4].
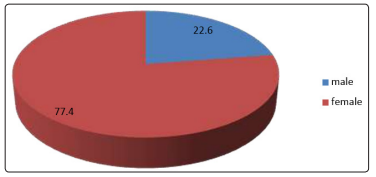
Figure 1: The Percent’s of the gender of the diabetic patients attending PHC centers in Khartoum locality, Sudan
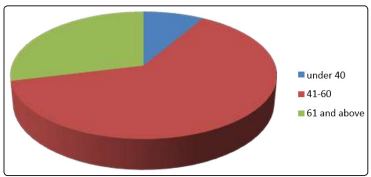
Figure 2: The Age of diabetic patients attending PHC centers in al Khartoum locality. (N=288)
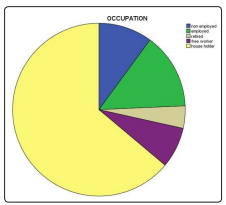
Figure 3: The occupation of the diabetic patients attending PHC centers in al Khartoum locality. (N=288)
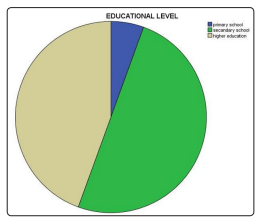
Figure 4: Educational level of the diabetic patients attending PHC centers in al Khartoum locality. (N=288)
Figure 5: Marital status of the diabetic patients attending PHC centers in al Khartoum locality. (N=288)
The association between the peripheral neuropathy and its risk factors was assessed using a T-test for continuous data and chisquare test for categorical data and the results were as follow: There is a significant association between the DPN and the increase in the age of the patient more than 58 years (p-value = 0.001) and increase the duration of diabetes for more than 10 years (p-value = 0.001). there is a significant association with the female gender (p-value = 0.002) also there is a significant association with the occupation of the patients: DPN is more prevalent in householder followed by the non-employed patients (p-value =0.001). [table 3]
There is a significant association between DPN and marital
status (p-value = 0.001). also, there is a significant association
between DPN and overweight patients (p-value = 0.001). there
is a significant association with the bad glycaemic control of the
patient (p-value =0.001). There is a significant association with
smoking (p-value = 0.012) and the type of treatment used by the
patient (p-value = 0.024). [Table 3].
On the other hand, no significant association was found between
the educational level of the patients (p-value = 0.127) and the type
of diabetes mellitus (p-value = 0.178). [table 3]
| DPN | Frequency | Percent |
|---|---|---|
| Present | 121 | 42 |
| Absent | 167 | 58 |
| Mean | SD | P value | ||
|---|---|---|---|---|
| Age | Present | 56.8 | 9.8 | 0.001 |
| Absent | 50.6 | 9.2 | ||
| Duration of DM | Present | 10.4 | 4.1 | 0.001 |
| Absent | 6.7 | 4.9 |
| Risk factor | Variables | Diagnosis of peripheral neuropathy | Total | P value | |
|---|---|---|---|---|---|
| Absent | Present | ||||
| Gender | Male | 27 | 38 | 65 | 0.002 |
| Female | 140 | 83 | 223 | ||
| Occupation | Employed | 12 | 27 | 29 | |
| unemployed | 35 | 6 | 41 | ||
| Retired | 4 | 8 | 12 | 0.001 | |
| Free worker | 7 | 15 | 22 | ||
| House holder | 109 | 75 | 84 | ||
| Educational level | Primary school | 7 | 9 | 16 | |
| Secondary school | 78 | 66 | 144 | 0.127 | |
| Higher education | 82 | 46 | 128 | ||
| Marital status | Single | 2 | 4 | 6 | 0.001 |
| Married | 158 | 98 | 256 | ||
| Divorce | 2 | 1 | 3 | ||
| Widow | 5 | 18 | 23 | ||
| Smoking | Smoker | 10 | 18 | 28 | 0.012 |
| Non smoker | 157 | 103 | 260 | ||
| Type of diabetes | Type 1 | 1 | 3 | 4 | 0.178 |
| Type 2 | 166 | 118 | 284 | ||
| Treatment used | Hypoglycaemic agents | 166 | 114 | 280 | 0.024 |
| Insulin | 1 | 3 | 4 | ||
| Both | 0 | 4 | 4 | ||
| BMI | Normal weight | 30 | 45 | 75 | 0.001 |
| Over weight | 135 | 75 | 210 | ||
| obese | 2 | 1 | 3 | 0.001 | |
| HbA1c level | Good control | 131 | 61 | 192 | |
| Bad control | 36 | 60 | 96 | ||
In gender: the odd of men developing DPN was 0.5 times lower the odds of female developing DPN and the odd of a smoker to develop DPN is 0.63 times lower the odd of the non-smoker. Regarding occupation of the patients, the odd of employed patients developing DPN is 2 times higher the odd of the householder developing DPN, and the odd of non- employed, retired, and free worker developing DPN was 0.4, 0.26, and 0.27 times lower the odd of the householder developing DPN, respectively. [Table 4].
Regarding educational level, the odd of the patient with primary education developing DPN was 0.33 times lower the odd of patients with higher education, and the odd patients with secondary education were 0.49 times the odd of patients with higher education developing DPN. [Table 4].
| P Value | OR | 95%CI | |
|---|---|---|---|
| Smoking | .362 | .633 | 237 - 1.692 |
| Male | .051 | .510 | .255 - 1.002 |
| Employed | 067 | 2.622 | .933 - 7.368 |
| Married | .009 | 4.183 | 1.420 - 12.323 |
| Divorce | .323 | 4.345 | 237 - 79.835 |
| Bad control | .000 | 3.102 | 1.806 - 5.328 |
| Over weight | .474 | .409 |
In the logistic regression model the bad glycaemic control and the marital status (being married) emerged as significant independent predictors for developing DPN (OR 3.102, 95% CI = 1.805-5.328, P = 0.001) and (OR=4.183, 95%CI=1.420 - 12.323, P=0.009) respectively
Regarding the type of diabetes mellitus, the odd of patients developing DPN was four times higher the odd of patients with type I DM developing DPN although there was no association (p-value = 0.178). The odd of Patients with normal weight and overweight developing DPN were 0.2 and 0.4 times lower the odd of obese patients developing DPN, respectively. [Table 4].
This study showed a high prevalence of DPN 42% based on the MNSI questionnaire and 10 monofilament testing. A study conducted in Iran used the same method for diagnosing peripheral neuropathy and it concludes that the combination of MNSI questionnaire and monofilament test are the most accurate screening tool for diagnosing PN in diabetic patients. This high prevalence resembles a systemic review and metaanalysis conducted across Africa which showed that the overall prevalence of diabetic peripheral neuropathy was 46% [30,37]. The current study shows a high degree of DPN compared to other studies in Saudi Arabia 35% and this may be due to the method for diagnosing PN in this study which diagnosed using a questionnaire (Douleur Neuropathique 4) and there is other studies showed lower prevalence include: Jordan 39.5%, Europe 28%, Qatar 34.5% and UK 28.5%, the difference in prevalence across the different country may due to different study population, study design and different method for diagnosing PN in diabetic patients [28, 29, 39, 32, 36].
On the other hand, a very high degree of DPN is estimated in
Tanzania 72.2% and this may be due to the area of study which
was a tertiary hospital, and it’s more porn to recruit patients
with complications than PHC centers on the current study. These
different degrees of DPN are attributed to early diagnosis and
proper case management as well as different geographical areas,
different study populations, and different methods used to diagnose
peripheral neuropathy in diabetic patients [34].
The current study shows that high prevalence of peripheral
neuropathy associated with increased age with the mean age of
developing PN was (56.8 ± 9.7) years of age and was 4.1% aged
[20-40], 49.5% aged [41-59] and 46.2% [60-75] aged and the odd
of patients aged [60-75] developing DPN is 9 times higher the odd
of patients aged [20-40] developing DPN and the odd of patients
aged [60-75] developing DPN is 3 times higher the odd of patients
aged [41-59] developing DPN. This may be attributed to the fact
that with the increase of age there is some degree of loss of some
nerve fibers, results obtained from a study conducted by Peter A.
Also increase age consistent with increasing duration of diabetes which leads to an increase in the frequency of microvascular and macrovascular complications.
There is a high association with increased duration of diabetes
with the mean duration found to be (10.4 ±4.1) years since
diagnosis, which was consistent with many studies showed the
same association but with different mean of age and duration of
diabetes and this may be due to increasing duration increased the
susceptibility for developing more complications of diabetes, in
Qatar the mean age was (57.5 ±10.7) and the mean duration was
(13.6 ± 7.9) and in Jorden, the mean of age was (57.8 ±9.7) and the
mean duration of DM was (6 ±5.1), also multiple studies conducted
in Italy and Iran showed the same association. In contrast, a study
conducted in Saudi Arabia showed no association between the
development of peripheral neuropathy and the age and duration
of DM [28, 29].
Regarding the gender of the patients, this study shows a high
association with the female gender (p-value =0.002) and this may
be due to the body difference of the females; high lipid levels and
it’s scientifically approved that the effect of high lipid resembles
the effect of hyperglycaemia on the nerve damage. A unique study
conducted in the USA in both type I and type II patients it showed
that gender was associated with developing PN only in patients
with type II DM with female predominance not in type I [33].
Regarding the occupation of the patients, the current study shows
a high association with the occupation of the patients and it shows
that being employed increase the risk of developing PN twofold
than being a householder these may be due to increasing the stress
due to workload but this farther studying to assess the type of
work and the working hours. This was inconsistent with a study
conducted in Jordan it showed that being unemployed increases
the risk of developing PN.
The current study shows a significant association with the type
of treatment for DM (p-value
= 0.024) mainly using oral hypoglycaemic agents increase the
risk of developing PN in diabetic patients that may be because
hyperglycaemia causing microvascular and macrovascular
complications this resembles a result obtained in a study conducted
in Tanzania showed increasing the risk of developing PN with
patients using oral hypoglycaemic agents [34].
Regarding smoking, this study shows an association between
developing PN and smoking (p- value 0.012) and this may be due
to the fact that smoking diminished blood supply to microvascular
nerve cells and that will result in nerve damage. This result is
consistent with a result obtained from a study conducted in the
USA [high association in type I patients (p- value 0.001) and less
association in type II patients (p-value 0.01)] and it’s inconsistent
with results obtained from other two studies conducted in Saudi
Arabia and Kuala Lumpur that showed no association. This
association needs further assessment because the duration of
smoking and the daily average cigarette consumption hadn’t
been assessed [33].
This study shows a high association with glycaemic control of
the patients (p-value 0.001) that being in a good control decrease
the risk of developing PN (odd ratio=1.58), in contrast, different
studies had shown no association with the HbA1C level include
a study conducted in Iran, India, Kuala Lumpur, USA, and Qatar
[27, 29, 30, 35, 39].
Another factor was the BMI of the patients and it was found to
be highly associated with the development of PN (p-value 0.001)
and Patients with normal weight and overweight were 20.7% and
40.9% less likely to develop PN than obese patients, respectively
this may be since obesity impaired the glucose tolerance so worsen
diabetes and hyperglycaemia which increase the risk of nerve
damage. In contrast, a study conducted in the USA showed a high
association with BMI in type I DM patients only (p-value 0.001)
and no association in type II DM patients. Also, no association
with it found in other studies conducted in Saudi Arabia, Iran,
India, and Kuala Lumpur [27, 28, 30, 39].
The current study addressed two unique risk factors which are the
educational level and the marital status and the results showed that
there is a high association with the marital status (p- value 0.001)
and it showed that married and divorce patients were four-fold
more likely to develop PN than widow patients and single patients
were 96% less likely to develop PN than widow patients this may
be due to the small percent of widow patients in the study.
Regarding the educational level, no significant association is
found between the development of PN and the educational level
of the patients.
There is a limitation to this study. The sampling was not random so the generalizability of this study to the general population is restricted and the study type is cross-sectional which will not enable the researcher to assess the long term effect of the risk factors on the outcome. The study restricted patients come to PHC centers which are less likely to recruit patients with complications. Also, there may some technical errors during neurological examination but this is minimized by using the same person for all the examination. Another important limitation is the lack of assessment of hypertension and the lipid profile of the patients as being an important risk factor and the lack of assessment of other conditions that may lead to neurological problems like vitamin B12 and folic acid deficiency [22-26].
This study has identified a high prevalence of peripheral neuropathy in diabetic patients attending the primary health care centers in Khartoum locality. Another important finding in this study is the association of the development of DPN with female gender, increase age, increase duration of DM, BMI, poor glycaemic control indicated by HbA1C level, smoking, marital status especially being married and divorced, the occupation of the patient especially being employed and the medication used for DM especially using oral hypoglycaemic agents. On the other hand, the type of diabetes and the educational level not found to be associated with the development of peripheral neuropathy in diabetic patients [31, 32, 38 & 40].
This study finding could provide practical information for diabetic peripheral neuropathy for better health policy and basement for further researches.
Inform the decision-makers in government and the ministry of health about the seriousness of the situation and prepare a plan for setting health policies and developing a campaign to increase DM awareness, prevention, and management.
Making the neurological history and examination part of the routine visit of the diabetic patient. Intensive health education of the patients to be aware of the complication of DM and the way of prevention and that tight control of the glycaemic status can prevent neurological complications. Also the regular follow up can detect the early complication so further deterioration can be prevented.
Further studies in larger population size and with different diagnostic tools to check the accuracy and to evaluate the effect of more factors in the development of DPN.
