Author(s): Pankaj Singh Negi* and Prakhar Jain
This review address cisplatin-induced hearing loss and various strategies tested to prevent this adverse effect. Cisplatin ototoxicity molecular pathways are still being investigated. Through the action of several transporters cisplatin enters targeted cells in the cochlea. After it enters the cochlea once, is retained for months to years. Cisplatin can inhibit protein synthesis, cause DNA damage and generate reactive oxygen species that can lead to inflammation and apoptosis of outer hair cells, resulting in permanent hearing loss. To prevent cisplatin ototoxicity utilized antioxidants, transport inhibitors, G-protein receptor agonists, and anti-inflammatory agents. There are no Food And Drug Administration-approved drugs to prevent cisplatin ototoxicity. Based on the long-term retention of cisplatin in the inner ear, any approved medication should either prevent passage of cisplatin into the inner ear or have the capability to continually protect against ototoxicity for months to years.
Objective: “This study starts to explain why patients who receive the drug sustain hearing loss,” Although corticosteroids may help to mitigate the ototoxic side effects of cisplatin, there are complications associated with their systemic and prolonged use “This is very important, because as we come to understand how cisplatin related hearing loss occurs, over time we may figure out a way to block it, or at least diminish its effects.”
As the population of cancer survivors continues to grow, so does the importance of addressing their medical needs. Patients treated with cisplatin the chemotherapeutic drug, one of the common consequence of treatment is permanent hearing loss [1]. Cisplatin ototoxicity reported rates varies widely, so it is believed that at least half of all patients treated with cisplatin will suffer hearing loss [2]. As cisplatin is widely used, this rate of ototoxicity translates to hundreds of thousands of patients affected each year in the United States alone. From various researches clinical researchers found that, in both humans and mice, cisplatin found in the cochlea, the part of the inner ear that enables hearing, months and even years after treatment. The drug is eliminated from most organs in the body within days to weeks after being administered. Cisplatin, a platinum-based drug, is used for the treatment of many cancers, including bladder, ovarian, and testicular cancers. But similar platinum-containing drugs and cisplatin can damage the cochlea, leaving 40%-80% of adults, and at least 50-55% of children, with significant permanent hearing loss, a condition that can greatly affect quality of life. Studies have shown that increasing doses of cisplatin are correlated with increasing loss of outer hair cells (OHC), one of the two types of auditory sensory cells together with inner hair cells (IHC), leading to irreversible hearing loss. This side effect of cisplatin is especially pronounced in young children, with a tremendous impact on speech, cognitive, and social development. The symptoms linked with hearing loss in children may occur within hours after cisplatin administration; however, delayed ototoxicity can also occur years after. Recent studies have shown that cisplatin is retained in the tissues of the inner ear of mice and humans for much longer periods than was previously thought, with cisplatin detected months to years after the last administration [3-10].
Guinea pigs treated with cisplatin demonstrated shifts in compound action potential amplitude growth curves that were greater at the higher frequencies(11). They also were observed to have shifts in the cochlear microphonic amplitude growth curves that appeared to be smaller than those for the compound action potential. Distortion product optoacoustic emissions were reported to be diminished in cisplatin treated mice. Auditory brainstem responses in cisplatintreated animals demonstrate increased thresholds, with greatest effects in the higher frequencies [11-13].
Interaction of cisplatin with the cochlear antioxidant defense system. Cisplatin is converted to a cis-diammine chloroplatinumupon entering the cell cytoplasm. These reactive platinum species can react with molecular oxygen to generate superoxide which is detoxified by superoxide dismutase to hydrogen peroxide and oxygen. Hydrogen peroxide is further detoxified by catalase to water and oxygen. Cisplatin reactive intermediate readily bind to and oxidizes the antioxidant reduced glutathione to oxidized glutathione. Glutathione peroxidase consumes GSH to produce GSSG in the process of converting H2 O2 into H2 O. Glutathione reductase reduces GSSG to GSH by using the reduced from of nicotinamide adenine dinucleotide phosphate (NADP+), NAPDH, as cofactor.
The cochlea has several transport mechanisms that could influence the uptake of cisplatin. The copper transporter (Ctr1) is strongly expressed in tissues targeted by cisplatin, the organic cation transporter (OCT2) is expressed in the organ of Corti and stria vascularis. Pharmacologic blockade of this transporter protects against cisplatin ototoxicity. Mechano transduction channels may be involved in the uptake of cisplatin. Inhibition of Mechano transduction channels protects against cisplatin-induced damage to hair cells in zebrafish, and mutants lacking these channels were shown to be resistant to cisplatin-induced cell death in zebrafish [14-17].
Risk factors include young age children less than 5 years of age, male children, elderly patients, cumulative dose > 400 mg/m, noise exposure, combination with other ototoxic drugs including carboplatin, nutritional depletion and anemia, cranial irradiation, and genetic predisposition [18].
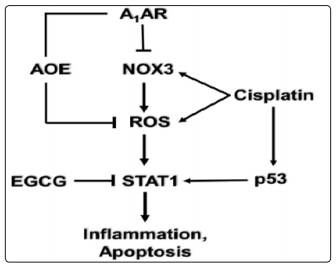
Mechanism of cisplatin-induced hearing loss, Adenosine receptor (A1AR)-dependent otoprotection. Cisplatin mediates NOX3 activation and reactive oxygen species (ROS) generation. The generation of ROS promotes signal transducer and activator of transcription1 (STAT1) activation which stimulates the inflammatory process. Activated STAT1 association with active p53 promotes the apoptosis of cochlear cells. The otoprotective effects of A1AR activation is mediated by reducing oxidative stress in the cochlea by activating antioxidant enzymes (AOE) and/or by suppressing the induction of NOX3. EGCG, a known inhibitor of STAT1, has been shown to protect against cisplatininduced hearing loss.
Studies described have identified a number of different mechanisms which mediated cisplatin hearing loss and provide the basis for rational drug design to treat this devastating condition. While we can say that most of the drugs which target these mechanisms are still in the experimental animal stage at the moment. These kind of studies need to be extended in human clinical trials for validation. And also there is routes of drug administration would be a major issue, as systemic administration of these drugs could compromise chemotherapeutic efficacy of cisplatin. In this regard, the use of a number of cisplatin transporters and antioxidants would have the greatest likelihood of interfering with cisplatin antitumor efficacy. Potential systemic toxicities can be eliminated by localized delivery of these drugs into the cochlea but in such case it would require an additional minor surgical procedure to achieve this goal. This can allow the use of a majority of the agents for protection against ototoxicity. Quite a few drugs are currently in clinical use or in clinical trials for other indications. These include TNF-α antagonists which are used for the treatment of chronic inflammatory diseases and EGCG which is in various clinical trials for the treatment of cancer. Such agents who are effective systemically could function as initial candidates for treating cisplatin-induced ototoxicity and other forms of hearing loss. Other drugs which could be progressive into clinical use are MET channel blockers, such as bulky aminoglycoside antibiotics, which demonstrate low potential for ototoxicity. Persistent research in this area would discover new mechanisms underlying cisplatininduced hearing loss and validate novel targets and drugs to treat this condition [19-27].
• Sodium Thiosulfate
• N-acetylcysteine
• Dexamethasone
• Amifostine
• Vitamin E
This article has discussed the targets and molecular and functional effects of cisplatin on cochlear function. Numerous preclinical investigations have been performed to ameliorate cisplatin ototoxicity. Clinical trials have yielded mixed results in some cases. However, promising phase 3 clinical trials with sodium thiosulfate administration have been reported. It is critically important to avoid interference with the chemotherapeutic efficacy of cisplatin when attempting to preserve hearing. The local treatment with intratympanic administration of protective agents is likely to avoid neutralizing the antitumor effectiveness of cisplatin and would avoid potential systemic toxicity of protective agents. Future innovations in drug delivery to the cochlea could provide novel methods to prevent cisplatin ototoxicity. This is an exciting area of clinical research and further breakthroughs are likely to appear in the near future [28-32].
In the guinea pig model the use of agents like fluticasone intracochlear implants is safe and able to provide effective otoprotection against cisplatin-induced hearing loss. The cochlea has a higher collective exposure to cisplatin than other organs and that, unlike other tissues, the cochlea has very little capacity for eliminating cisplatin. This may explain why the cochlea is predominantly prone to cisplatin toxicity. For clinical researchers working to develop a method of preventing cisplatin induced hearing loss, outcomes suggest that the best manner of preventing cisplatin ototoxicity may be via prevention of cisplatin uptake into the cochlea in the first place, As soon as inside the cochlea, cisplatin give the impression to be there to stay. Identifications of drugs that inhibit the uptake of cisplatin through the stria vascularis or directly bind or else inactivate cisplatin as it enters the cochlea may be a feasible path toward avoidance of cisplatin induced hearing loss. The results study underscores the importance of longterm hearing monitoring in patients who have received cisplatintherapy. Until clinical researchers make significant progress in preventing cisplatin ototoxicity in cancer therapy, it is critical treating cancer survivors with cisplatin.
