Author(s): <p>Delia Teresa Sponza</p>
In this study the photodegradability of tree micropollutants namely meprobamate (MEP), androstenedione (ADR) and estrogen (estrone (E1)) was investigated by a zinc oxide / zinc sulphide-biochar nanocomposite. SEM analysis results showed a porouse structure was detected in the surface nanocomposite when MP were not doped while its structure exhibited a crystal layer when the MPs were bound to the nanocomposite. XPS analysis showed characteristic peaks for Zn 2p, S 2p, C 1s, and O 1s while t The peaks near 1,026.88 and 164 eV were the binding points of Zn 2p and S 2p1/2, respectively. According to TEM results dark points was nor detected on the surface of the biochar while to these locations was reached at the upper layers of the biochar. EDS data exhibited a heterogen layer between C, O, Zn and S elements.The maximum photodegradation yields were 89%, 89 and 99% for MEP, ADR and E1 micropollutants at a increased from 45%, 53 and %60% up to 89%, 89 and 99%at a zinc oxide / zinc sulphide-biochar nanocomposite concentration of 2 mg/l at a zinc oxide to zinc sulphide ratio of 1 to 2 and at a pH of 7.00 after 15 min phottoxidation time. The produced nanocomposide can be reused 16 times with the same yields for micropollutants. After 17. Run the yields decreased only %1
Micropollutants (MPs) are synthetic or naturally occurring in the environment and cause adverse ecological and or human health effects (1-7). These pollutants are usually organic compounds that persist in the environment because they do not biodegrade. The MPs and their metabolites can be personal care products, detergent, cosmetics, fragrance, natural and synthetic hormones, fire retardants, surfactants, plasticizers, antimicrobial compounds, endocrine disrupting compounds, ultraviolet absorbing compounds, domestic products and nanotechnology residuals [8-10]. These compounds are typically detected at low concentrations, usually in the range of nanogram per liter or microgram per liter. These compounds are “xenobiotic compounds” because most of these compounds are of anthropogenic origin. They are trace organic compounds because their low concentrations [11-15]. Micropollutants such as human and veterinary pharmaceuticals and their metabolites, natural and synthetic endocrine disrupting compounds are usually excreted via urine and feces and can subsequently enter into the aquatic environment through municipal wastewater treatment plant effluents [16-17].Wastewater treatment plant effluents have been identified as an important point of discharge for MPs into the aquatic environment since WWTPs are not typically designed to remove MPs. The limited removals of MPs that have been reported from wastewater treatment processes since the concentrations of MPs exist in the WWTP influent are too low. The complex chemical structure of some MPs also enhances their persistence and recalcitrance during wastewater treatment. The concern about MPs has been accentuated by reports of gonad and Tertiary wastewater treatment technologies such as advanced oxidation process (using UV, hydrogen peroxide or ozone) and membrane technologies (using MBR, nano-filtration and reverse osmosis) have been reported to compared to conventional treatment methods [18-22]. However, there are concerns of producing more toxic oxidation by-products or metabolites during the use of advanced oxidation process to treat wastewater. These tertiary treatment processes are energy intensive, resulting in increased operational cost to the plant owners. Therefore, the optimization of the operation of existing biological wastewater treatment processes to remove MPs is desirable however is not possible.
Semiconductors (e.g., Ti02 , ZnO, Fe2 03 , CdS and ZnS) can act as sensitizers for light-induced redox processes due to their electronic structure, which is characterized by filled valence band and an empty conduction band [17-20]. When a photon with a energy of hv matches or exceeds the band gap energy, Eg, of the semiconductor, an electron, is promoted from the valence band, VB, into conduction band, CB, leaving a hole, hvb+ behind. Excited state conduction-band electrons (e"ob) and valence-band holes can recombine and dissipate the input energy as heat, get trapped in meta-stable surface states, or react with electron donors and electron acceptors adsorbed on the semiconductor surface or within the surrounding electrical double layer of the charged particles [20-26]. In the absence of suitable electron and hole scavengers, the stored energy is dissipated within few nanoseconds by recombination. If a suitable scavenger or surface defect site is available to trap the electron or hole, recombination is prevented and subsequent redox reaction may occur. Most organic photodegradation reactions utilize the oxidizing power of the holes directly or indirectly; however, to prevent a buildup of charge one must also provide a reducible species to react with the electrons. In contrast, on bulk semiconductor electrodes only one species, either the hole or the electron, is available for reaction due to band bending. However, in very small semiconductors particle suspensions both species are present on the surface [27- 30]. Therefore, careful consideration of both the oxidative and the reductive paths is required.
In this study it was aimed to degrade meprobamate (MEP), androstenedione (ADR) and estrogen (estrone (E1)) using zinc oxide/ zinc sulphide-biochar nanocomposite via photocatalysis process. The surface of zinc oxide / zinc sulphide-biochar nanocomposite produced under laboratory conditions was investigated with SEM, XPS, TEM and EDS. The effects of increasing zinc oxide / zinc sulphide-biochar nanocomposite concentrations, zinc oxide to zinc sulphide ratios, pH variations, and the presence of some cation and anions on the photodegradation yields of MEP, ADR and E1 were studied. The reusability properties of the zinc oxide / zinc sulphide-biochar nanocomposite was researched.
A glass reactor with a volume of 2 liter was used for experimentation. The solar ligth was used as UV source with a UV power of 25 W. The samples were taken from a surface water. The studies performed at 21°C. The studied meprobamate (MEP), androstenedione (ADR) and estrogen (estrone (E1))doses, zinc oxide / zinc sulphide-biochar nanocomposite concentrations, zinc oxide to zinc sulphide ratio, photodegradation times, and Ph ranges were mentioned in the results and discussion section of the paper.
The samples extracted for MP analysis were initially spiked with surrogate standard solution (100 µL of 0.5 ppm) of the selected compounds prepared in methanol before the extraction. The samples extracted for characterization by the YES assay did not include the spiked standards. Visiprep solid-phase extraction (SPE) vacuum manifolds coupled with Oasis® MAX SPE 6 mL (500 mg) SPE cartridges were used to concentrate the filtered samples. The HLB tubes were conditioned with 6 mL of methanol, 6 mL 0.1M NaOH in Milli-Q water, and 6 mL of Milli-Q water. The filtered surface water sample was loaded onto the cartridge at a flow rate of approximately 5 mL/min to ensure good recovery and the sample bottles were rinsed with 10 mL of pH 8 distilled water. After loading, the cartridges were rinsed with 2 mL of pH 8 water and dried under vacuum for 10-20 minutes. The compounds were then eluted from the cartridges with 2 mL of MeOH at a flow rate of about 1 mL/min and rinsed thrice with 3 mL of 2 % formic acid in methanol at a flow rate of 1 mL/min, waiting six minutes between each elution. After elution, the cartridge was aspirated to dryness for about 10 min under vacuum. The eluted fractions were collected in amber glass tubes and dried under a gentle stream of nitrogen gas (N2 ). The dried residue was dissolved in 0.4 mL of methanol and stored at 4°C before using it for YES assay or LC-MS-MS analysis.
The morphology of the biochar and zinc oxide / zinc sulphidebiochar nanocomposite were investigated using SEM. The abundant pore structure of the biochar surface without micropollutans was illustrated ?n Figure 1a and b. When the surface of the zinc oxide / zinc sulphide-biochar nanocomposite was loaded with micropollutants some rigid crystals with different sizes was illustated in Figure 1c and d.
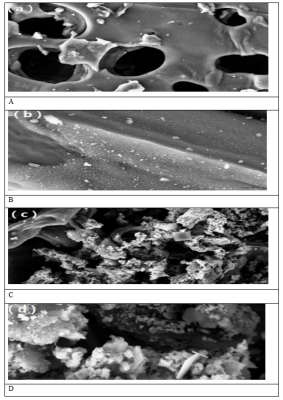
Figure 1: SEM Analysis Results of Zinc Oxide / Zinc SulphideBiochar Nanocomposite Undoped (a,b) and Doped (c,d) with Micropollutants
These analysis were performed to detect the element characterization and the value of the chemicals in the zinc oxide/ zinc sulphide-biochar nanocomposite. The total spectrum of the zinc oxide / zinc sulphide-biochar nanocomposite was illustrated in Figure 2. Some characteristic peaks of Zn 2p, S 2p, C 1s, and O 1s was detected. This nanocomposite have Zn, S, O, and C elements. The peaks near 1,026.88 and 164 eV were the binding points of Zn2p and S2p1/2, respectively. Zinc oxide/ zinc sulphide have larger O 1s peak area near 539.0 eV and this results with an aggregate of ZnS and ZnO2 . The dark locations in the biochar was found to be zinc oxide and zinc sulphide. The diameter of the zinc oxide / zinc sulphide was measured as 1,78 nm with an rectangular morphology (data not shown).

Figure 2: XPS Analysis in the Zinc Oxide / Zinc SulphideBiochar Nanocomposite (a) Zn 2p, S 2p, C 1s, O 1s (b) Zn 2p ½ and Zn 2p 3/2, c) C1 s and C1 d) O 1 s and O1 e) S 2p ½ and S 2p 3/2
It was not found dark locations on the surface of the biochar as seen in Figures 3a and b. Dark locations was detected as illustrated in Figures 3c and d in the upper layers of the biochar.
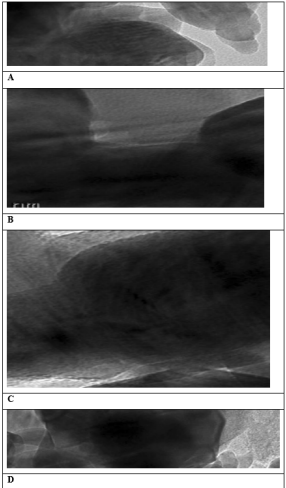
Figure 3: TEM Analysis Results (a,b) without Dark Locations, (c,d) with Dark Layers
EDS Analysis Results EDS exhibits the chemical ingredients of the zinc oxide / zinc sulphide-biochar nanocomposite( Figure 4). This figure illustrates the presence of C, O, Zn and S cations and the presence of C, O, Zn and S in the surface of zinc oxide / zinc sulphide-biochar nanocomposite. This disturbances exhibits a heterogen structure.
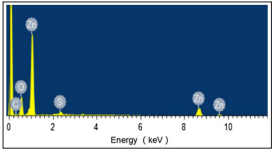
Figure 4: EDS Analysis Results the Presence of C, O, Zn and S in the Studied Nanocomposite
As the zinc oxide / zinc sulphide-biochar nanocomposite concentrations were increased from 0,5 up to 2 mg/L the photocatalytic removals of MEP, ADR and E1 micropollutants increased from 45%, 53 and60% up to 89%, 89 and 99% ( Figure 5). Further increase of nanocomposite concentration up to 5 mg/l did not affect the micropollutant yields since all the active sites of the zinc oxide / zinc sulphide-biochar nanocomposite were fully covered by the micropollutants and non of point of the surface of the aforementioned nanocomposite did not produce OH radicals under solar ligth [25-30].
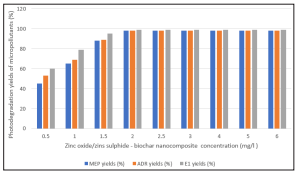
Figure 5: Effect of Increasing Zinc Oxide / Zinc Sulphide-Biochar Nanocomposite Concentrations on the Photodegradation of Micropollutants after 15 min Contacting Time
The zinc oxide to zinc sulphide ratios were adjusted as 0.4 to 1, 1 to 1, 2 to 1, 3 to 1, and 4 to 1 in the 2 g zinc oxide / zinc sulphide-biochar nanocomposite concentration. Te zinc oxide to zinc sulphide ratio of 1 to 2 has the best photodegradation yields for MEP, ADR and E1 yields under a solar ligth of 27 W. The maximum photodegradatiin yields were 98%, 98% and 99% after 15 min photodegradation ( Figure 6). At the highest zinc oxide the activated sides forming OH radicals decreased on the surface of the zinc oxide / zinc sulphide-biochar nanocomposite and lower catalytic active sites remained. Thus lowered the photodegradation of micropollutan
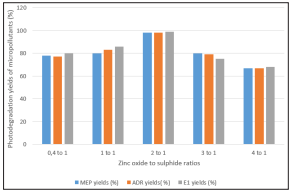
Figure 6: Effects of Zinc Oxide to Zinc Sulphide Ratios to the Photodegradation Yields of Micropollutants in the Nanocomposite after 15 min Photodegradation Duration
As the pH was increased from 2 to 6 and 7 the photodegradation yields of MEP, ADR and E1 increased from 20-35% up to 90-99% (Figure 7). Under acidic and alkaline conditions photocatalytic degradation rate was signifcantly reduced. During photodegradation catalytic activity was closely related to the charged nature of the surface of the catalyst. Te pH primarily afected the rate of photodegradation by changing the electrostatic interaction of the photocatalyst surface by affecting the OH radical production in the charged nanocomposite.
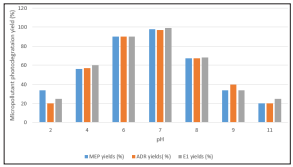
Figure 7: Effect of pH on the Photodegradation Yields of Micropollutants
The effect of some cations ( Na+ , Fe2+, Cu2+, and Zn2+) and some anions ( SO4 2-, NO3 -, Cl- and CO3 2-) on the photodegradation yields of the MEP, ADR and E1 micropollutants were investigated. The concentration of the anions and cations were adjusted to 0,02 mg/l. Fe2+ , Cu2+, and Zn2+ improved significantly the ptotodegradation yields of micropollutants. Na+ had the lowest positive effect ( Table 1). All the anions improwed the photodegradation yields. The anions in this study was not change the electrostatic interaction between micropollutants and zinc oxide / zinc sulphide-biochar nanocomposite.Terefore, OH radical production was pozitively affected by these cations and anions.
Table 1: Effects of Some Cations and Anions on the Photodegradation Yields of Mep, Adr and E1 Micropollutants
| Micropollutant names | Without cation and anions 1 mg/l zinc oxide / zinc sulphide-biochar nanocomposite yield (%) | Fe2+, yield (%) | Cu2+, yield (%) | Zn2+, yield (%) | Na+ , yield (%) | SO4 -2 , yield (%) | NO3 - yield (%) | Cl-, yield (%) | CO3 2-, yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| MEP | 83 | 93 | 90 | 88 | 89 | 94 | 92 | 87 | |
| ADR | 82 | 96 | 93 | 90 | 90 | 94 | 95 | 93 | |
| EI | 84 | 96 | 94 | 93 | 90 | 95 | 96 | 94 |
Reuse of zinc oxide / zinc sulphide-biochar nanocomposite
Reusability of the zinc oxide / zinc sulphide-biochar nanocomposite was studied to detect the resistance of this nanocomposite. The photocatalytic studies were repeted 15 times. The photodegradation yields showd that after 16 runs the photodegradation yields reduced 1% (Figure 8). Th?s showed that the studied nanocomposite exhibited high stability and perfect reusability under ultraviolet ligh during photodegradation.
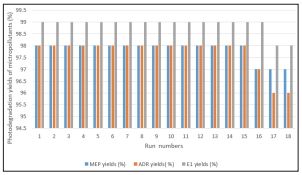
Figure 8: Reusability Studies of Zinc Oxide / Zinc SulphideBiochar Nanocomposite for Micropollutant Removals
The results of this study showed that the micropollutants namely meprobamate (MEP), androstenedione (ADR) and estrogen (estrone (E1)) can be effectively photodegraded with a nanocomposite namely zinc oxide/ zinc sulphide-biochar produced under laboratory conditions. The highest photocatalytic capability was achieved with a at a zinc oxide / zinc sulphidebiochar nanocomposite concentration of 2 mg/l at a zinc oxide to zinc sulphide ratio of 1 to 2 and at a pH of 7.00. By supporting of Zinc oxide / zinc sulphide on the surface of biochar, the active area was enlarged, which enhanced catalytic activity ending with 89%, 89 and 99% MEP, ADR and E1, removals,respectively. The resusability of the zinc oxide / zinc sulphide-biochar nanocomposite was excellent. After 17 runs the photodegradation yields decreased only 1%. Terefore, ZnO/ZnS@BC is a good material for the catalytic degradation of NOF in water and has favourable application prospects in sewage treatment systems.
