Author(s): <p>Oscar Solís Salgado* and Ana Ivette Bahena Olivares</p>
The Virchow-Robin perivascular spaces (VRPVS) are microstructures that are part of the large weave of vessels penetrating the deep white matter of the brain and spinal cord; together with other cellular components, they help maintain central nervous system homeostasis.
In the initial work, these vascular structures are described as dura mater invaginations going towards the deep white matter of the brain and the spinal cord. Thus, they are the first contributions to their micro-structure. Actually, the relationshop between these vascular structures and other cellular and molecular components is relevant because of their contribution of nutrients as well as the elimination of toxic substances from the central nervous system.
Thanks to this progress we may now understand the physiopathology of neurodegenerative diseases, where there is special damage of the brain’s deep white matter as a result of a malfunction of the now well described Virchow-Robin perivascular spaces.
This work starts with the history of perivascular spaces (PVS), analyzing them from their microstructural discovery up to their present micro-anatomical description. This establishes the bases for an understanding of the glymphatic system, which drains toxic substances from the brain, among other components of the interstitial fluid (ITF).
Understanding the functioning of the VRPVS and providing the bases for a definition, is central to comprehending the physiopathology of brain diseases. Since scarce knowledge exists on these structures, this study is a contribution to clinical practice so that personnel in the neurosciences may have a better foundation to understand them.
Los espacios perivasculares de Virchow-Robin (VRPVS) son microestructuras que forman parte del gran tejido de vasos que penetran en la sustancia blanca profunda del cerebro y la médula espinal; junto con otros componentes celulares, ayudan a mantener la homeostasis del sistema nervioso central.
Basado en los trabajos iniciales, se describe a estas estructuras vasculares como invaginaciones de la duramadre hacia la sustancia blanca profunda del cerebro y médula espinal, conformando así las primeras aportaciones a su microestructura, y en la actualidad la relación de estas estructuras vasculares con otros componentes celulares y moleculares, tienen una gran relevancia en el aporte de nutrientes y a la vez en la eliminación de sustancias tóxicas para el tejido nervioso central. Gracias a este avance se puede entender ahora la fisiopatología de enfermedades neurodegenerativas donde implica en especial un daño a la sustancia blanca profunda del cerebro producto de la disfunción de los ahora bien referidos espacios perivasculares de Virchow-Robin.
Este trabajo parte de la historia de los espacios perivasculares (EVP), analizándolos desde su descubrimiento microestructural hasta su descripción microanatómica actual. Esto sienta las bases para una comprensión del sistema glinfático, que drena sustancias tóxicas del cerebro, entre otros componentes del líquido intersticial (ITF).
Comprender el funcionamiento del VRPVS y proporcionar las bases para una definición es fundamental para comprender la fisiopatología de las enfermedades cerebrales. Dado que existe escaso conocimiento sobre estas estructuras, este estudio es un aporte a la práctica clínica para que el personal de las neurociencias pueda tener una mejor base para comprenderlas.
Durand Fardel, in 1842, first noted the existence of the Virchow- Robin spaces (VRS) or état criblé [1]. However, what he really described was an enlarged VRS of the periarteriolar spaces in post-mortem brains and referred to their appearance in the ganglia at the base of the brain. In 1849, Johann Heinrich Pestalozzi described the VRS for the first time, as invaginations of the dura mater containing cerebrospinal fluid (CSF), following the cerebral arteries towards the parenchyma, without providing any other anatomical fact about this structure [2].
In 1851, Rudolf Ludwig Karl Virchow described a microscopic space between the external lamina (tunica adventitia), the internal and middle laminae (tunica intima and tunica media) of the cerebral blood vessels. He called it “Diseziierende Ektasie” (dissecting ecstasia) and in his published findings he described it as a space without interruptions between the vascular laminae [3].
In 1859, Charles Philippe Robin confirmed these contributions and was the first to describe the perivascular spaces (PVS) as normal cerebral channels. He proposed that PVS are connected to the perineural spaces (which are a part of the glymphatic drainage channels). However, he differed from Virchow in considering these channels to be closed at both ends (cerebral parenchyma and subarachnoid space (SAS)). Both Robin and Virchow provided important anatomical descriptions of these spaces, which became eponymous of the true cerebral perivascular spaces [3].
In their research, Key and Retzius, in 1875, described funnel- shaped spaces - “Piatrichter” - which are formed in the cerebral cortex, connecting to the SAS and surrounding the blood vessels that penetrate the cerebral parenchyma through tissue that is continuous with the leptomenix [4, 5].
In 1894, Tuke considered the perivascular sheaths to be solely derived from the pia mater and to represent the adventitial layer of cerebral blood vessels. He stated that these sheaths surround the blood vessel system up to the capillary level, providing them with a perineural capsule that comes from the pia mater extensions [4].
This knowledge was followed by contributions from Mott, in 1910, who was the first one to suspect that there could be differences in the extension of the perivascular layer of the veins, compared to the cerebral and spinal arteries; thus, he considered it to be a better and more noticeable perivascular layer around the arterial system [4].
The original concept of the relationship between the cerebral PVS and the CSF within the SAS, was first described in physiological studies published by Weed in 1923, at Johns Hopkins University. In these investigations, the pia mater was seen to extend around the PVS, from the cortex towards the cerebral parenchyma [6, 7].
Some authors proposed the existence of a direct or free communication between the VRS and the SAS; in spite of discrepancies in the interpretation of Weed’s work, histological and ultrastructural studies mainly seemed to support the idea of a free communication between the SAS and the VRS [6].
In 1958, Woollam and Millen distinguished between two layers that formed the PVS, which are [5]:
On the other hand, Millen and Woollam’s studies (1961 and 1962) showed that the PVS have free communication with the SAS. Like this, they reinforced Virchow and Weed’s previous research [5].
In 1962, Nelson et al. described the PVS as a space around the blood vessels, which is internally delimited by elements of the tunica media or middle layer, and externally by the basal glial membrane. These results helped consolidate even more the studies carried out by Millen and Woollam, describing the external and internal layers forming the VRS [5].
By 1974, Cloyd and Low observed that the internal lining of the VRS was separated by intercellular gaps. They proposed that the CSF may enter through these fenestrations towards the PVS, which are continuous [5].
Based on scanning electron microscopy (SEM) studies carried out by Hutchings and Weller in 1986, it was observed that the pia mater seems to be an intact cell plaque that goes from the cerebral or spinal cord surface towards the subarachnoid vessels, thus separating the SAS from the subpial and perivascular space [7, 8].
Doubts about the free communication between the two spaces (SAS and VRS) were cleared thanks to experiments with tracers such as horseradish peroxidase, albumin or another radiolabeled tracer injected into the brains of rats. These substances were found in the PVS of the middle cerebral artery (Bradbury, Cserr and Westrop, in 1981, and again by Cserr, in 1984 - 1988). These were groundbreaking experiments using radiolabeled tracers to understand their movement in the CSF and ISF perivascular route, as presently shown by Jeff Iliff [6].
A low recovery of isotopic tracers was observed in the CSF volume. These results suggest that the cerebral ISF does not drain directly into the SAS CSF, but follows a perivascular route along the large cerebral vessels within the SAS [6].
An anatomical description of the SAS - VRS separation has been established, thanks to several studies in mammals, including in human beings [6].
It has been shown that the pia mater in the brain’s surface and in the spinal cord connects to the surfaces of blood vessels found in the SAS; like this, the VRS is separated from the subpial space in the SAS [6].
However, the anatomical models established for animal brains and human brains did not correlate exactly with results from tracer studies in rats, as described in 1984 [6].
1. There was no confirmation of a direct connection between the perivascular spaces (PVS) of the cerebral vessels and the PVS of the vessels at the SAS level. With the models published up to that moment, there was a belief that the fluid or tracers were dissipated in the subpial space, more than draining directly within the PVS of the subarachnoid vessels [6].
2. No distinction had been made between the PVS of arteries and veins in order to quantify, in a preferential manner, the concentration of tracers found in the arterial PVS [6].
Finally, Alcolado, in 1988, carried out an investigation in humans, on the cranial arachnoid mater and the pia mater - anatomical and ultrastructural observations where he sets forth that the PVS and SAS are continuous with the subpial compartment and parenchymatous PVS. This takes place through small fenestrations in the pial layer; previously, in 1976, Peter, Palay and Webster had stated that the PVS were communicated with the SAS and that at the capillary level, the VRS tended to disappear because of the junction of basal membranes (BM) and the glial endothelium [7].
For many years now, there has been acceptance of the fact that there is direct anatomical communication between the SAS and the VRS at the level of the cerebral surface and the spinal cord. This evidence is supported by and is a result of the use of light microscopy and, in the last century, electron microscopy.
In 1954, Woollam and Millen presented in their work a view of the vessels entering the brain from the SAS as having an extension of the arachnoid cover and a layer of pia mater. This leptomeningeal extension was described as a reticular perivascular layer, broadly corresponding to the pia-glial membrane (Schaltenbrand and Baildy) [9]. This PVS is the eponymous “Virchow-Robin space.”
The space between the blood vesssels and the nervous tissue was described as a vessel that entered or left the cerebral or spinal cord surface.6 Initial observations, using the scanning electron microscope, clearly determined that the pia mater was a cell cover which connected with the vessels in the SAS, more than a cover that follows the vessel itself within the cerebral cortex. This anatomical conformation leads to direct communication between the SAS and the VRS, as suggested by the preliminary studies [9].
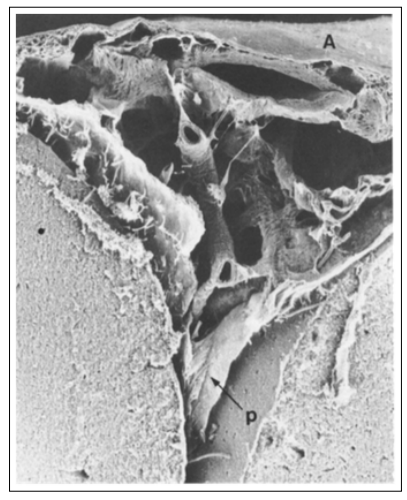
Figure 1: The external arachnoid (A) layer may be seen horizontally in the upper part of the figure. The pia laminae (p) of the lateral walls of the groove partially detach from the underlying cortex. A subarachnoid vessel in the center of the figure is joined to the arachnoid by thin cords and wider trabeculae.
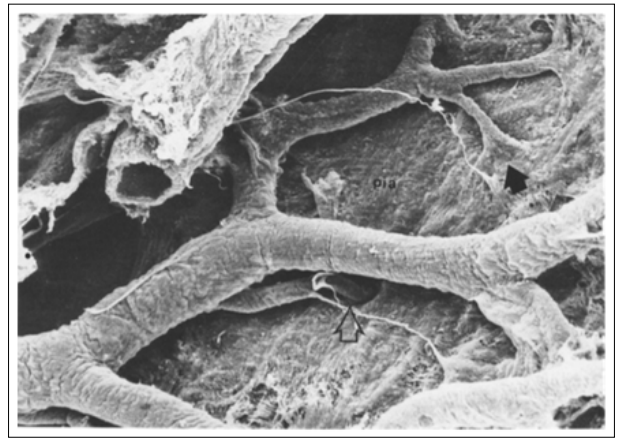
Figure 2: The leptomeningeal external layer of the vessel fuses with the pia mater as a fan-like structure (black arrow). A branch of a vessel in the center of the image seems to pass through a deep hole (open arrow), which is probably an invagination of the pia. The thin cords run between the pia and the vessels.
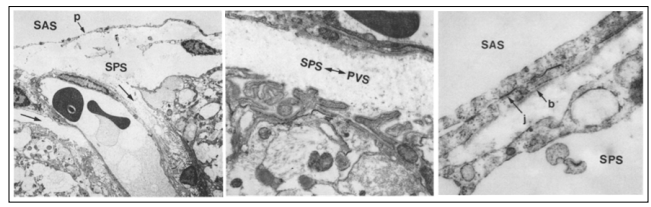
Figure 3: Above and to the left: the entrance to a small blood vessel on the surface of the normal cerebral cortex. A continuous pia mater (p) layer separates the subarachnoid space (SAS) from the subpial space (SPS). While the vesicles extend over the cortex, the subpial space extends along the outside of the vesicles as the perivascular space (black arrows). Above and to the right: Higher magnification TEM of the region marked below the two long arrows in the upper left-hand side. The irregular surface of the cortical glia limitans may be seen in the lower part of the figure and its basal membrane is separated from the wall of the vessel by the subpial perivascular spaces (SPS-PVS) which have scarce collagen fibers.
The pia mater (figure 4 below), consists of one to three layers of cells, creating a barrier that is formed by intercellular junctions, many of which have a morphology of gap junctions or desmosomes. In this way, the pia mater forms a regulatory interface between the SAS and the brain’s surface with the VRS [9, 10].
The scanning electron micrographs of the leptomeningeal brain vessels in a normal human being, in animals and in spinal cord, show that the pia mater forms a cover that goes from the brain surface over the blood vessel that runs through the SAS. The pia mater is continuous with the meningeal cover of the vessel. This anatomical constitution of the pia mater separates the SAS from one end of the subpial space and the other end of the perivascular cortical space [9, 10].
If the pia mater forms a barrier between the SAS, the underlying subpial space and the perivascula space, it is extremely valuable to know the permeability characteristics of this barrier [9].
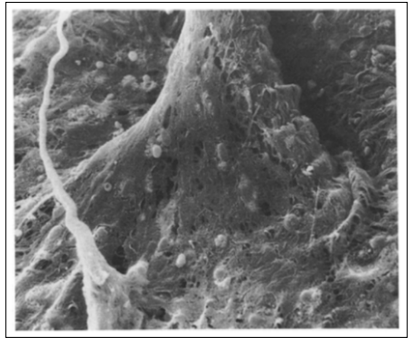
Figure 4: The pia mater forms a fan-like structure when it spreads over the artery’s surface
Anatomically, the VRS are defined from the histological and structural point of view, as a space that surrounds the blood vessels (arteries, arterioles and venules) when these penetrate from the SAS towards the depth of the brain parenchyma. Initially the idea prevailed that the VRS are connected to the SAS, allowing a free communication of fluid. It has been suggested that the ISF may be drained out along these channels towards the SAS and eventually towards the arachnoid villi [11].
Later on, according to red light microscopy observations, it can be seen that the PVS behave as “cul-de-sacs”, open towards the SAS, but closed towards the brain parenchyma. Like this, there is no channel per se for the flow between these spaces.
The first systematic study of blood vessels that enter the cerebral cortex, done with electron microscopy, confirmed this question. It has been reported that small arterioles enter the brain cortex carrying within them (at the point where they turn into capillaries) an extension of the SAS. Presently these findings show that the obliteration of the VRS in the capillary bed leads us to reconsider theories about the existence of a peri-capillary CSF circulation (the fourth circulation) [11].
The VRS are more complex. Presently, their fine structure is built on endothelium, over the pia mater, layers of glial cells, and each one is outlined by different BM [11].
The glial membrane (glia limitans) converges in the cerebral parenchyma, forming an external wall of the VRS [11].
In the capillary bed, the BM of the glia fuses with the external vascular membrane; like this, it obstructs the VRS.
The pial sheath creates a space that is near the vascular wall, which is called the PVS.
At the entrance site, the vessel that is found within the VRS joins its pial cover with the layer of pial cells that cover the brain surface forming a funnel-like structure which accompanies the vessel within the the VRS in the brain parenchyma.
Nevertheless, the pial cover of the artery, but not of the veins, extends within the VRS [11].
Near the capillary bed, the pial cover becomes more and more fenestrated and then disappears. It is important to note that the nomenclature is not used in a consistent manner. Some authors use the term VRS and PVS as synonyms, while other use the terms differently [11].
In ultrastructural electron microscopy studies, it is stated that the pia mater separates the VRS from the cortical SAS, in spite of the fact that the layer of pial cells separates the VRS from the cortical SAS. There is strong physiological evidence showing fluid circulation along the VRS (Figure 2).
The CSF of the SAS is contained between the arachnoid mater and the pia mater. The leptomeningeal blood vessels go through the CSF of the SAS on the cerebral surface. These vessels are covered by a layer of leptomeningeal cells, sometimes called the cell layer of the adventitia. Under the pia mater and its own BM, the subpial space can be found, which contains connective tissue, such as collagen, macrophages and subpial blood vessels [12].
Studies suggest that the VRS are continuous with other blood vessels in the SAS, the subpial space and the parenchyma; they are separate from the subpial space and the perivascular space of the cerebral parenchyma through the glia limitans, which is composed of layers of astrocyte feet and, and of their own associated BM [12].
Within the brain parenchyma, cells such as neurons, oligodendrocytes, microglia, astrocytes and associated processes, create a narrow and winding extracellular space that is filled with ISF [12].
The VRS is surrounded by different cell components depending on the level at which it is found, as may be seen in a cross section [12].
The adventitial space is generally called the perivascular subarachnoid space and is surrounded by a layer of leptomeningeal cells which separates the blood vessel wall and that of the VRS from the CSF. It has potential entry points (stoma, intercellular grooves and possibly other structures) [12].
A capillary vessel in the brain consists of endothelial cells, pericyte, astrocyte feet and their respective basal membranes. At the capillary level it is not clear if the endothelium BM - pericyte and astrocyte BM are completely fused or open to some extension, such as for example, the existence of a pericapillary space, which could potentially allow for communication along the VRS of the entrance to the vascular tree of an arteriole to capillary and from this to venule [12].
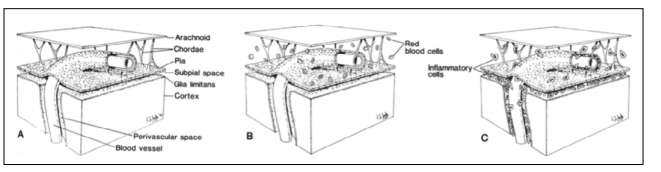
Figure 5: The diagrams are based on data derived from this study and show the link in the normal brain (A), in the brain of patients who died from subarachnoid hemorrhage (B) and in the brain of patients who died from leptomeningitis (C).
The leptomeningeal blood vessel system which ends in the depth of the brain and of the venous system, stems from the interior cerebral parenchyma on its way to the cortical surface of the encephalon. It follows a route that starts in several compartments, travelling from the SAS, where the leptomeningeal arterial vessel system begins, and goes to the subpial space; here it finally turns into vessels that penetrate the cerebral parenchyma, ending in the capillary bed of the deep white matter [12, 13].
The following is a description of anatomical aspects of the vascular system, the different compartments of its trajectory and its anatomical relationship to the VRS.
The leptomeningeal arterial system is born in the main arteries of the brain, starts at the SAS level and then penetrates the cerebral parenchyma and contributes an arterial irrigation system. This leptomeningeal arterial system has anatomical and functional importance since it finally forms a cerebrovascular functional unit.
Just as the perivascular system, the leptomeningeal system has different components depending on the level of the anatomical section that is seen from the cortical surface until its depth.
We have divided this system into the following components:
1. Subarachnoid space
2. Subpial space
3. Perivascular Virchow-Robin space
Before going into the anatomical description of the leptomeningeal arteries in the three compartments, we must remember that the main structure for CSF production is the choroid plexus, ependymal cells and, to a lesser degree, the inside of the cerebral parenchyma. This CSF starts circulating within the frontal horns of the lateral ventricles, going through the Monro foramen, aqueduct of Sylvius, fourth ventricle, and draining through the Luschka and Magendie holes at the craneal base, towards the SAS, then reaching the convexity and being absorbed by the arachnoid villi that drain into the superior sagittal sinus (SSS). The CSF that goes through the SAS towards the depth of the encephalon, to function as a glymphatic pathway in the clearance and replacement of CSF with ISF and solutes, goes down this pathway through the leptomeningeal vessels and the different components of the VRS [12].
The leptomeningeal artery in the cerebral surface has three different layers; from the inside out, these are:
1. Intima: Consists of endothelial cells, BM of endothelial cells and internal elastic lamina [12].
2. Media: Formed by few layers of smooth muscle cells in a circumference-like arrangement and their own BM [12].
3. Adventitia: Formed by dispersed connective tissue, especially collagen and cells that are dispersed in this layer, some of them formed by macrophages and, finally, by fluid that is no more than the SAS fluid that forms the CSF [12].
We must highlight the fact that the adventitial space or adventitial layer of the leptomeningeal artery in the SAS, is not strictly speaking the adventitial layer of a peripheral artery; this concept refers to the space that encloses the artery (subarachnoid) and is surrounded by a layer of leptomeningeal cells which separates the wall of the blood vessel and at the same time, the VRS in the other compartments of the CSF itself that encircle it. This anatomical structure shows different points of entry as fenestrations or stomas, intercellular grooves and possibly, other structures. The adventitial layer is formed by pial cells, macrophages, fibroblasts and collagen, which help provide stability to the leptomeningeal arteries in the SAS [12].
The structures reported in the Zervas investigation (1982) are uniform fenestrations with well defined and smooth edges, as well as diameters in the range of micrometers but which at the same time, form a structure that is constituted by collagen fibres that give it a mesh-like property and separate the SAS from the VRS. In this study we observed an adventitial layer with stomas, similar to what had been previously reported; for this reason we concluded it is a round or oval shaped anatomical structure, joined by different bonds such as desmosomes and hemidesmosomes. The stomas reveal that they have a mesh of underlying connective tissue and strands of collagen that are anatomically visible between their edges [12].
The blood vessels of the cerebral cortex surface extend into the SAS and towards the subpial space. As we said before, a leptomeningeal artery of the SAS has a complete internal elastic lamina/lamella and a cover of smooth muscle cells. The layer of pial cells that form the adventitial layer continues through trabeculae into the pia mater itself [12].
Small pores or gap unions are observed in the pial cover but the adjacent cells are joined by desmosomes and small nexus junctions [12].
The arterioles within the subpial space are smaller than the arteries in the SAS and lack a complete internal elastic lamina. The subpial arterioles are completely lined with a thin layer of pia mater cells. This cell cover is completely separate from the overlying pia mater and the surface of the cerebral cortex. The individual cells are joined by nexus junctions and also have true desmosomes between the cells at this level, occasionally the pial layer of a small arteriole or of a meta-arteriole. In the subpial space they seem to be sustained from the deep surface of the pia mater itself [6].
The middle layer of these arteries at the subpial level is composed of two cell layers of smooth muscle and surrounded by a very thin layer of pial cells, joined by nexus junctions. At the same time, the pial layer is separated from the wall of the vessel by a collagen layer [6].
The great arterioles of the cerebral cortex and the subcortical white matter (deep white matter) are surrounded by two or three layers of smooth muscle, as well as the arterioles in the subpial space, which lack a complete internal elastic lamina [6].
Fully surrounding this arteriole, we find a diminished layer of flat cells joined by nexus junctions, which are similar to pial cells and separate the cover of smooth muscle cells from the glia limitans that surrounds them [6].
In glia, pial cells and underlying smooth muscle cells, there is little connective tissue and space between these cell layers. At the arteriolar level, they have very small fenestrations. Since the number of smooth muscle cells that surrounds the arterioles decreases as these go deeper into the white matter, at the same time the size of the fenestrations in the pial layer increases [6].
In general, the pial cells that surround the small arterioles and meta-arterioles are associated with the smooth muscle cells, just as in other species; the VRS around the capillaries are obliterated by fusion of the endothelium and the glial BM. At this level pia mater cells cannot be seen around the capillaries [6].
1. Subarachnoid space
2. Subpial space
These venous blood vessels in the brain surface, in the same way as the arteries, extend from the subpial space to the SAS, and then drain into the large venous sinuses [6].
One vein in the SAS is also covered by a thin lamina of pial cells, which is separated from the endothelium by a layer of collagen, flat cells and has a surface of smooth muscle cells with dense characteristics. The cells of the venous pial cover are joined by desmosomes and by small nexus junctions. The large veins in the subpial space are frequently surrounded by layers of connective tissue composed of collagen and with a random orientation. This layer of connective tissue is similar to the one seen around the SAS veins [6].
Frequently, the small veins within the subpial space have scarce connective tissue around them and do not have a continuous pia mater perivascular cover, compared to the arterioles of the subpial space, but instead are surrounded by groups of pia mater cells [6, 14].
Rarely, the small venules in the subpial space are covered by more layers of collagen. This collagen arrangement and the rudimentary stimulus from cells that arise from the deep part of the pia mater, suggest that this vessel seems to be a small trabecular vein when going through the subarachnoid compartment [6].
The venules in the cerebral cortex and in the white matter have relatively large lumens with respect to the thickness of their vacular wall, when compared to arterioles and meta-arterioles. The vascular wall has flat endothelial cells and an incomplete layer of atypical smooth muscle cells that are similar to the ones seen around the veins in the cat’s brain. A narrow and regular VRS separates the venule wall from the BM of the glia limitans and contains a small number of collagen fibres. No complete layer of leptomeningeal cells is seen around a venous vessel, at the level of the cerebral cortex or of the superficial white matter [6].
For many years these terms have been used by some authors in an inconsistent manner and referring to the same structures, without giving them the anatomical importance corresponding to each one of these words. For this reason, we redefined the terms “perivascular” and “paravascular” with respect to the glymphatic system.
It is important to highlight the fact that our objective is to correctly establish the meaning of both terms according to their etymology:
1. “Peri” prefix: means around, enclosed or surrounded [15].
2. “Para” preposition: comes from the Latin “pro ad” (in front of); others say it comes from “per ad” (for). It is also a Greek prefix, or similarly, a preposition used as a proverb (?) (next to, along, against, to the side of). But this isn’t fully used for circumference-type structures [15].
For this reason, we believe it is important to redefine and anatomically conceptualize each one of the relevant points for a good functioning and understanding of this structure as a perivascular Virchow-Robin space. Thus, it must be studied according to its anatomical and ultrastructural levels in the different structures and anatomical layers that are found when penetrating more deeply into the parenchyma (penetrating arteries) or when leaving (veins) the deep white matter.
The first description of the Virchow Robin perivascular spaces, was made by Durand Fardel in 1842, and the present anatomical- functional description is by Jeff Iliff; these have revolutionized knowledge on certain neurodegenerative diseases, as well as on tumors and inflammatory diseases.
In 1851, the great German pathologist Rudolf Ludwig Karl Virchow first made a microscopic description of these spaces (structural), with the cerebral vessel penetrating the deep white matter through the cerebral cortex. This description was based on two fundamental aspects: a space that surrounds a penetrating blood vessel, which is separated from the vessel itself by its vascular lamina (adventitia), with no interruption. By 1859, Charles Philippe Robin described the perivascular spaces as normal cerebral microstructures.
The next contribution to these structures was to consider them similar to a funnel shape, communicating with the subarachnoid space (SAS), and for the first time the description was provided of the leptomeningeal tissue surrounding the penetrating blood vessel (continuity of leptomeninges).
In 1894, Tuke considered that the external vascular layer (perivascular) of each penetrating vessel derives from the pia mater (which constitutes the adventitia), and a second statement to the effect that the lining follows the penetrating blood vessel until it turns into a capillary vessel. After this structural limit, the lining disappears.
Mott, in 1910, first analyzed the microstructural differences of the perivascular layer in leptomeninges. This analysis was based on the extension or area covering the veins, with respect to the arteries, thus marking a notable difference between the leptomeningeal layer between veins and arteries, which is better structured at the arterial level.
Weed, at Johns Hopkins University, in 1923, originally refers to the relationship maintained by the PVS-CSF within the SAS itself. Later on, much research would be done on this microstructural- functional relationship and thus, the bases were established for the glymphatic system, led by Iliff.
Weed, at Johns Hopkins University, in 1923, originally refers to the relationship maintained by the PVS-CSF within the SAS itself. Later on, much research would be done on this microstructural- functional relationship and thus, the bases were established for the glymphatic system, led by Iliff.
The controversy as to whether there existed or not direct and free communication between the VRS and the SAS itself was established by Weed. Microscopy structural studies described by Woollam and Millen in 1958, were based on the premise that the layers constituting the PVS are formed by the pia mater (external layer) and the arachnoides, covering the penetrating blood vessel (internal layer). Besides, the expected assertion as to a free communication between the VRS and the SAS was also confirmed.
In 1962, Nelson and collaborators stated that these perivascular spaces have two great limits: an internal one, formed by the tunica intima or innermost layer, and the external one, formed by the basal or glial membrane; this established even more the bases for an internal and an external layer of the VRS.
Cloyd and Low, in 1974, stated that the internal layer of the VRS has intercellular fenestrations that would allow transit of CSF, as was confirmed by the existence of the glymphatic system.
Scanning electron microscopy allows for visualization of the pia mater extension that reaches the deep white matter, accompanied by the penetrating blood vessels, making the separation noticeable between the SAS an the subpial and perivascular space.
The free communication between the two spaces (SAS and VRS) was confirmed with the experiments using radio tracers injected into animal brains and within the CSF. This was clearly described by Cserr since 1982 and, finally, by Jeff Iliff, who presented the perivascular route from the CSF and ITF within the glymphatic system.
Finally, Alcolado, in 1988, confirms the structural and functional communication between the VRS and the subpial compartment, by the presence of fenestrations of the pial layer that is wrapped around the penetrating blood vessel in the cerebal parenchima or the spinal cord.
The eponymous perivascular Virchow-Robin space (PVVRS) is a microstructural component with hystological and functionalcharacteristics surrounding a blood vessel. It must be seen as a function of its contribution to the homeostasis of the nervous system.
Our contribution is to establish in a clear and precise manner, the term “perivascular” to the term “paravascular”, which is used for the VRS, and thus universally describe these microstructures [16, 17].
Declaration of Funding: The authors of this article did not receive any financial support to carry out this work.
Conflict of Interest: The authors of this article have no conflict of interests.
