Author(s): Kevin T Moriarty, John H Rundback* and Christopher Shackles
Purpose: Percutaneous deep venous arterialization (DVA) using a stent-graft system is a novel endovascular approach in the treatment of patients with Buerger’s Disease.
Case Report: A 30-year-old male patient diagnosed with Buerger’s Disease presented with a painful, non-healing medial left hallux wound with ulceration. Lower extremity angiogram revealed extensive occlusion of all three tibial vessels in a pattern consistent with Buerger’s disease. Initially, revascularization and angioplasty of the posterior tibial artery was performed to supply flow but was occluded after one week, despite the patient being treated with aspirin and low molecular weight heparin. Since there was no suitable surgical target for bypass, the decision was made with the patient to perform deep venous arterialization to intervene. Percutaneous DVA was implemented to restore blood flow and to allow wound healing. The patient subsequently had a planned distal left hallux amputation which was non-healing. The decision was then made to perform a transmetatarsal amputation which then demonstrated complete surgical wound healing.
Conclusion: A successful posterior tibial DVA ultimately resulted in resolution and revascularization. Deep venous arterialization serves as an alternative option to prevent above ankle amputation, to heal wounds, and to improve quality of life in patients with Buerger’s Disease.
While DVA has been investigated for its successful use in the treatment of no-option critical limb ischemia patients with Rutherford classifications of 5 or 6 in the PROMISE 1 Trial and in the ALPS Multicenter Study, this technique has never before been investigated for the treatment of patients with Buerger’s disease [1, 2]. Buerger’s disease, also known as thromboangiitis obliterans, is a rare non-atherosclerotic inflammatory disease that affects small to medium sized vessels of the extremities which is characterized by ischemic rest pain, peripheral artery disease, Raynaud’s phenomenon, ulceration of distal digits, and significant vessel stenoses. Angiographic findings consisting of corkscrew collaterals around areas of segmental occlusion are one of the diagnostic criteria for Buerger’s disease. The exact cause of Buerger’s disease is unknown. However, Buerger’s disease is highly prevalent among young male cigarette smokers, and it often affects the lower leg vasculature significantly [3, 4]. The procedure of deep venous arterialization to create an intentional stent supported arteriovenous fistula between selected tibial arteries and veins with the goal of establishing retrograde perfusion to wounds has been shown to be a promising option for revascularization of the lower limb [5]. Procedural details of DVA creation have been previously described [6]. Although in our experience we have used “off-the-shelf” commercially available devices for this procedure [7]. We herein report successful outcomes in a patient with Buerger’s disease following DVA.
A 30-year-old male presented with a 9-month history of a painful non-healing medial left hallux wound attributed to a history of Buerger’s disease with secondary Raynaud’s phenomenon with a history of moderate tobacco use in college and occasional marijuana use since. Initial arterial imaging shows monophasic flow into the foot and below ankle arterial occlusive disease. The patient also has a history of hypertension and a prior spinal cord stimulator implanted for the management of digital pain. Initial physical exam reveals ruboric discoloration and pain in the left foot with a Wagner grade 2-3 exudative wound approximately 2cm in diameter over the medial aspect of the hallux (Figure 1). The right foot is slightly cool with diminished capillary refill and there are weak Doppler DP and PT collateral signals in both feet. Mild edema is noted bilaterally.
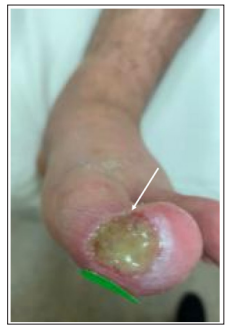
Figure 1: Initial consult demonstrating left medial hallux wound with ulceration
The patient underwent left lower extremity angiogram which revealed complete proximal occlusion of all tibial arteries with numerous corkscrew collaterals in a pattern consistent with Buerger’s disease (Figure 2). This was treated utilizing orbital atherectomy, angioplasty and a proximal coronary drug-eluting stent in the posterior tibial artery with restored flow into the foot. Tacks (Intact Vascular, Wayne, PA) were also placed in the medial plantar artery for flow limiting dissection following angioplasty. Completion angiography demonstrates brisk wide patency of the posterior tibial artery into the medial plantar artery directly out to the wound base. The patient was started on cilostazol as well as rivaroxaban and aspirin.
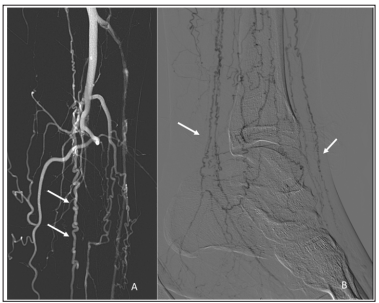
Figure 2: Left lower extremity angiogram demonstrating complete proximal occlusion of all tibial arteries (A) with arrows demonstrating numerous corkscrew collaterals in a pattern consistent with Buerger’s disease (A&B).
The patient returned 2 weeks later without clinical improvement, and duplex arterial imaging shows re-occlusion of the posterior tibial artery. The wound was noted to be slightly worse than baseline (Wagner grade 2 or 3, measuring approximately 1.5 to 2cm in greatest diameter), and the patient had continued severe pain. Since it was felt that the patient now had non-reconstructible (“no-option”) arterial disease and no suitable target for distal bypass, a DVA was performed. From both femoral and pedal accesses, angiography was again performed showing recurrent total occlusion of all tibial arteries and no flow through the recently revascularized posterior tibial artery. Using “off-the shelf” devices, a posterior tibial artery to posterior tibial vein DVA was created. A 5mm nominal diameter by 10 cm long Viabahn stent graft (Gore, Tempe, AZ) was used for the arteriovenous flow reversal, with completion arteriography demonstrating a widely patent posterior tibial deep venous arterialization with brisk flow into the foot and through the pedal venous arch.
One-month post procedure the patient was noted to have persistent but improved toe pain with granulation of the medial hallux wound, a warm foot and excellent capillary refill. Strong doppler signals were noted in the DVA and through the deep venous arch. Due to wound extension to the tendon the patient underwent distal hallux amputation, and hyperbaric oxygen therapy was started for wound healing. Two months later, the patient presented with an ischemic first toe amputation site with Duplex evidence of stenosis within the DVA stent graft (Figure 3). Repeat angiography was performed and a stenosis at the proximal DVA stent edge was successfully treated with drug-coated balloon angioplasty and additional stent graft placement which restored brisk patent perfusion to the patient’s first toe amputation site as well as the remainder of his forefoot (Figure 4).
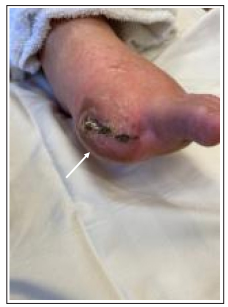
Figure 3: At 3 months post-DVA, a secondary wound infection resulted in additional angioplasty of the DVA stenosis and amputation at the first metatarsal phalangeal (MTP) joint of the left hallux. Restored brisk patent perfusion to the patient’s first toe amputation site as well as the remainder of his forefoot is demonstrated post-DVA revision
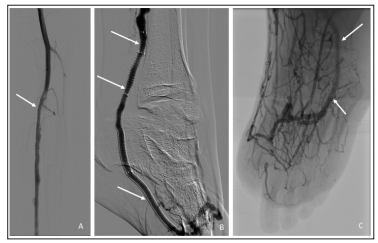
Figure 4: Completion arteriography demonstrating a widely patent posterior tibial deep venous arterialization (A). Stent extension and brisk flow into the foot (B) and through the pedal venous arch are demonstrated (C). Interval amputation for an unsalvageable left hallux is demonstrated (C).
Follow up at one month demonstrated a non-healing first toe amputation site and forefoot for which the patient was seen for wound care. The decision was made with the patient to perform a transmetatarsal amputation (TMA) of the forefoot as the best option to allow full healing. Follow up at two months post procedure demonstrates a healed TMA with approximation of the surgical site after suture removal (Figure 5). The patient is currently pain free and ambulatory.
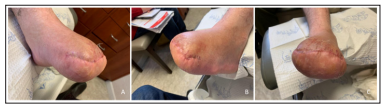
Figure 5: Patient underwent transmetatarsal amputation (TMA) and received wound care. Healing and approximation of surgical site demonstrated from medial (A), lateral (B), and anterior (C) perspectives.
Thromboangiitis obliterans (Buerger’s disease) is a pan vasculitis associated with small vessel arterial occlusive disease and a high rate of limb amputation [4]. Therapy is predicated upon discontinuation of active smoking and optimized medical therapy with HMG-CoA reductase inhibitors, antiplatelet therapy, vasodilators, phosphodiesterase inhibitors, calcium channel blockers, analgesia, possible sympathetic nerve block, intermittent pneumatic compression, and careful foot care [8, 9]. Revascularization for patients with Buerger’s disease has been described with variable outcomes [10, 11]. In a recent study by Modaghegh et. al., patients with Buerger’s disease who developed critical limb ischemia were treated with percutaneous transluminal angioplasty (PTA) with a limb salvage rate of 92% supporting endovascular treatment of Buerger’s disease [12]. In a study by Ghoneim et. al., a prospective study was conducted on the feasibility and effectiveness of endovascular procedures in 39 patients with Buerger’s disease presenting with critical limb ischemia. 15 patients underwent percutaneous transluminal angioplasty (PTA), another 15 patients underwent medical treatment, 4 patients underwent surgical bypass, and 5 patients underwent lumbar sympathectomy. The 12 month follow up showed 66.7% technical success in the endovascular group with 46.7% patency rate, 86.7% limb salvage rate, and 66.7% clinical improvement. Despite these results, endovascular treatment of Buerger’s Disease is challenging because of the prevalent location of lesions in distal vessels especially of the lower extremity, with compromised run-off at the foot level, making reconstitution of distal arteries (dorsalis pedis, plantar, and foot arch) mandatory to achieve success [9]. In patients with Buerger’s disease presenting with chronic limb-threatening ischemia (CLTI) in whom revascularization is not possible or successful, an above ankle amputation is often necessary [13].
DVA is a novel technique that establishes retrograde venous perfusion to the ischemic lower extremity by creating a stentgraft supported communication between tibial arteries and veins. In patients with atherosclerotic arterial disease, there is often an absence of involvement of venous structures, so that redistribution of antegrade flow through the veins may provide a patent conduit and directed flow to the foot. In Buerger’s disease, while there may be concomitant venous inflammation, it would still be expected that venous patency is relatively preserved even with advanced arterial disease involvement [14]. Consequently, DVA represents an attractive potential therapeutic strategy for patients with Buerger’s disease and non-reconstructible arterial disease or failed prior arterial revascularization. Prior results with DVA in patients with atherosclerotic disease have demonstrated amputation free survival between 67% and 80% at one year [1, 2, 15]. Reinterventions to preserve both anatomic and clinical patency were reported in 37% of patients for which types and patterns of DVA failure were categorized and intervention strategies proposed [7].
DVA represents a novel treatment option but has not been reported in the treatment of inflammatory vasculitides including Buerger’s disease. In our experience, the initial DVA creation and revascularization presented in this case of Buerger’s disease was successful, with follow-up at one month demonstrating effectiveness in improving perfusion and promoting wound healing with the presence of granulation tissue and capillary refill at the left hallux. At 3 months post-DVA, a secondary wound infection resulted in additional angioplasty of the DVA stenosis and amputation at the first MTP joint, demonstrating the importance of close conduit surveillance and consistent with the potential need for reintervention seen in prior studies. The patient then underwent a necessary transmetatarsal amputation (TMA) which demonstrated healing and resolution of the wound two months post procedure. The potential need for reintervention following the use of DVA will be further investigated in the treatment of Buerger’s disease.
In summary, DVA represents a potentially attractive treatment option in refractory Buerger’s disease patients who would otherwise require a major above ankle amputation. Similar to prior experience, DVA failure and reinterventions are common and mandate close graft surveillance and reintervention to maintain patency and wound perfusion. Further studies in a larger patient population would be beneficial to investigate the use of DVA and intervention strategies for the treatment of Buerger’s disease.
