Author(s): <p>Théophile Perrod*, Asmahane Benmaziane, Karine Aure, Laure Ladrat, Nassiba Heba and Jaafar Bennouna</p>
ABSTRACT
Neurological Immune Related Adverse Events (irAEs) secondary to Immune Checkpoint Inhibitors (ICI) are unncommon with an incidence of 1% to 5%. Among them, the Guillain Barré syndrome may be life-threatening and must be diagnosed early. We describe a clinical case of Guillain Barré Syndrom with a concomitant Campylobacter Jejuni colitis occurring after the first intravenous injection of pembrolizumab. A review of literature regarding the Guillain Barré syndrome after ICI use is added as a complementary source.
Pembrolizumab, a humanized immunoglobulin G4 monoclonal antibody belongs to the Immune Checkpoint Inhibitors (ICI) family. Pembrolizumab interacts with the receptor PD-1 expressed on lymphocytes, blocking the link with its ligand PD-L1, then releasing the reactivation of cytotoxic T-cells [1, 2].
ICI are well tolerated with few grade 3-4 Immune Related Adverse Events (IR AEs). According to the ESMO guidelines 2022, the most frequent toxicities are Immune Related Cutaneous AEs (IRcAEs), 50% all grades, but rarely severe, followed by IR- endocrinopathies, i.e hypothyroidism (6-9%), hypophysitis (1 %), and adrenal insufficiency (1-2 %). Other toxicities are observed, such as diabetes mellitus with insulin dependance (1-2%), and hepatitis (grade 3 1%-2%) [3]. Overall, the toxicity rates increase when anti-PD-1/PD-L1 antibodies are delivered in combination with anti-CTLA-4 antibodies, such as ipilimumab.
The incidence of neurological Immune Related Adverse Events (irAEs) is low with a range of 1%-5%. A large variety of neurological irAEs have been described, including encephalitis, aseptic meningitis for the central nervous system but also acute or chronic demyelinating polyneuropathy, cranial nerve neuropathy, myasthenic syndrome and myositis for the peripheral nervous system [3, 4].
ESMO’s guidelines 2022 mention that neuromuscular disorders account for approximatively 50% of neurological irAEs, which primarily pool myositis, myasthenia gravis, demyelinating polyradiculoneuropathy and overlapping syndromes [3]. Immune related neuropathies are mostly demyelinating and may present as an acute polyradiculoneuritis (Guillain Barré Syndrome) with an incidence of 0.2%-0.4%. Anti- ganglioside antibodies are usually described as negative [3]. According to ESMO’s guidelines, CSs (corticosteroids) are initially recommended and associated with a favorable outcome. If corticosteroids are unsuccessful, Intravenous Immunoglobulin (IVIG) is an additional or alternative treatment. A permanent discontinuation of ICI is highly recommended in case of grade 3-4 IR neurotoxicity [3].
In this report, we present the clinical case of a patient with Guillain- Barré syndrome after treatment with one dose of pembrolizumab for a microsatellite instability-high (MSI-H) locally advanced duodenal adenocarcinoma.
A 69-year-old man was hospitalized for anemia caused by a digestive bleeding. A gastro esophageal endoscopy showed a hemi- circumferential ulcerated lesion with budding edges localized in the duodenal bulb. Biopsies were in favor of a moderately differentiated adenocarcinoma with a concurrent loss of MLH1 and PMS2 immunoexpression.
A CT-scan evaluation confirmed the locally advanced disease without metastatic dissemination. Surgery was contraindicated due to unstable angina and moderate to severe calcific aortic stenosis. According to MSI status, a first intra-veinous infusion of pembrolizumab 200 mg was performed at day 1. At day 18, the patient suffered from grade 3 diarrhea with more than ten stools per day, a weight loss of 8 kg in three weeks and grade 3 asthenia according to Common Terminology Criteria for Adverse Events (CTCAE). There was no fever. An abdominal CT-scan showed colitis with symmetric, diffuse thickening of the rectum, sigmoid,and ascending colon. Recto-sigmoïdoscopy was not done. No stool culture was done. However, a Campylobacter Jejuni serology was in favor of a recent infection, as confirmed by the presence of IgG (37,15U/ml) and IgM (70U/ml).
Diarrhea was treated with intravenous (IV) methylprednisolone 2mg/kg per day, loperamide and IV hydratation. At day 28, the patient exhibited progressive proximal tetraparesis and distal paresthesia leading to the diagnosis of severe Guillain-Barré syndrome as the patient had lost the ability to walk. The ONLS (Overall Neuropathy Limitations Scale) score was at 11/12. There was no respiratory impairment. An electroneuromyography showed a large decrease of motor nerve amplitudes with discrete demyelinating abnormalities contrasting with sensitive nerve amplitudes conservation raising an acute motor axonal neuropathy (AMAN).
Serum antibodies against gangliosides, AntiGM1 (IgG), antiGD1a (IgG and IgM) and antiGT1b (IgG) were positive.
Intravenous immunoglobulin, 2mg/kg for 3 days and intravenous corticosteroids (IV methyprednisolone 240mg for 5 days) were administrered, followed by oral prednisolone 80mg per day. Pembrolizumab was definitively interrupted.
An electroneuromyography was done three weeks later and concluded to a worsening of the sensitive and motor amplitude especially for the lower members and stability for the upper members. The CT-scan made three months later, knowing that pembzolizumab was definitively interrupted, unfortunately showed an increase of the duodenal lesion and a dissociated response on the lymph nodes. There was no new metastatic lesion observed.
At 4.5 months, the neurologic evaluation concluded to an asymmetric improvement of the motor symptoms in favor of the right side. A 2/5 motor deficit on the left levator muscles of the leg and 4/5 on the flexor muscles. Hip flexion was impossible on both sides. On the upper limbs, a 4/5 motor deficit persisted on the left arm. An electroneuromyography was made and concluded to a moderate improvement of the sensitive and motor amplitude on the four limbs and a weak detection on multiple muscles including the proximal muscles.
Guillain-Barré syndrome (GBS), an acute peripheral neuropathy, may be associated with permanent or long-term sequalae and occasional fatality. Acute infectious illnesses precede 50%-75% of the GBS cases. Although many infectious agents have been associated with GBS, the strongest documented association is with Campylobacter infection. The association was first reported by Rhodes and Tattersfield in 1982. In a systematic review of published studies, approximately 30% of cases of GBS had evidence for preceding infection with Campylobacter jejuni. In terms of pathogenesis, activated macrophages and T cells and serum antibodies against gangliosides are observed but their significance is unclear [3-8].
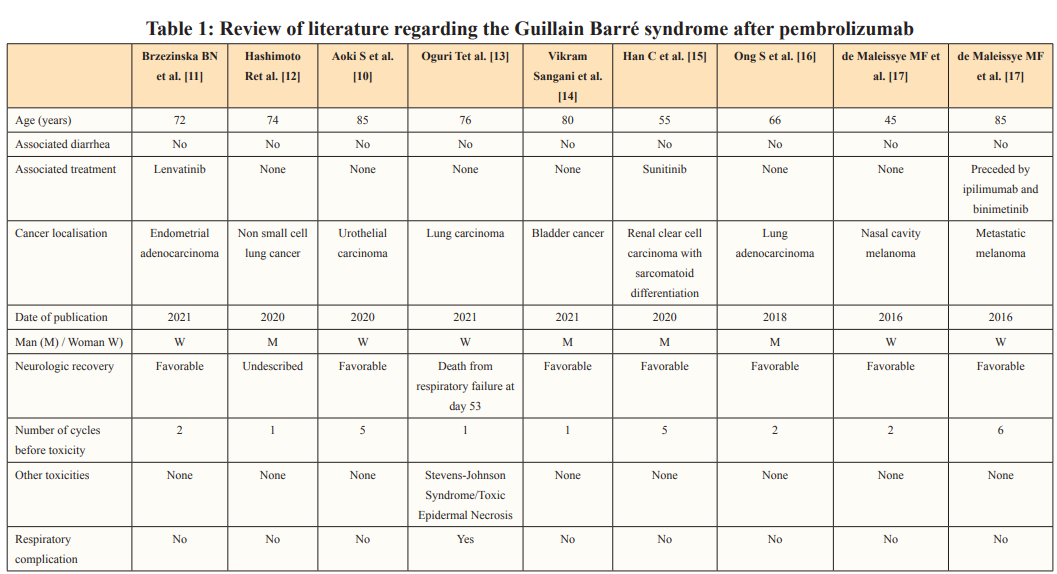
In our knowledge, few cases were published reporting Guillain- Barré syndrome or Guillain-Barré like-syndrome induced by pembrolizumab Table 1. One case was associated with Stevens- Johnson Syndrome leading to death after respiratory complications [9]. All cases occurred at the beginning of the treatment: from the first to the fifth injections. Guillain-Barré syndrome was also described with others ICI such as nivolumab Table 2.
Most of the time, the ICI was used as monotherapy Table 1. All patients were treated with corticosteroids and intravenous immunoglobulins except for one patient who received immunoglobulins and plasma exchange. Acyclovir was prescribed in one case because of a concomitant Ramsay Hunt syndrome. Neurologic recovery was variable and uncertain. Tumoral response to ICI, when described, was observed and prolonged, emphasizing the concept of high efficacy of ICI after grade 3-4 related toxicities. One case described by Aoki et al., showed a complete response of a stage IV urothelial carcinoma 6 months after the last dose of pembrolizumab [10].


The originality of this present case is the clinical sequence of pembrolizumab administration followed by Campylobacter Jejuni colitis and Guillain-Barré syndrome. Campylobacter is otherwise known to cause acute motor axonal neuropathy which is an axonal classification of Guillain-Barré syndrome. Diarrhea with colitis is one of the major adverse events observed with ICI, anti-PD-1 or PD-L1 and anti-CTLA4. According to the ESMO grade 3-4 colitis should be explored by stool testing for enteropathogenesis, Clostridium Difficile toxins, faecal calprotectin or lactoferrin, whole blood PCR for cytomegalovirus, complete blood count, serum C-reactive protein and electrolytes, interferon-gamma release assay, hepatitis A, B, C and E end human immunodeficiency virus tests and endoscopic evaluation. ICI therapy is recommended associated with IV methylprednisolone 1mg/kg/day switched to prednisolone 1mg/kg/day after 3 to 5 days.
Because of its scarcity, no data was available regarding re- introduction of ICI after immunotherapy-induced Guillain-Barré syndrome.
In 2019, Gravbrot et al [28]. reported a case of a 71-year-old gentleman who developed a Guillain-Barré after three cycles of ipililumab (cytotoxic T lymphocyte antigen-4 inhibitor) in adjuvant therapy for stage IIIB melanoma. Ipilimumab was discontinued and few months later, he was treated with pembrolizumab for a recurrent disease. A complete response was observed with pembrolizumab, and no toxicity was reported [29, 30].
Immune-related adverse events (irAEs) involving the nervous system must be known by medical oncologists because of growing use of immunotherapy in solid tumors. In this case, a Guillain-Barré syndrome was observed after Campylobacter Jejuni colitis occurring after one cycle of pembrolizumab. It underlines the absolute necessity to early manage gastro-intestinal immune toxicities in order to avoid rare life-thretening neurological toxicities.
The authors have no conflict of interest to declare. Written informed consent was obtained from the patient to publish this report in accordance with the journal’s patient consent policy.
1.Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236: 219-242.
2.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252-264.
3.Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, et al. (2022) Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up5, J Ann Onco l33: 1217-1238.
4.Heesung Moon, Seul-Gi Kim, Seung Ki Kim, Jinkwon Kim, Seung Ryeol LYWM (2022) A case report of re-challenge of immune checkpoint inhibitors after immune-related neurological adverse events: Review of literature. Medicine (Baltimore), J Immunother Cancer 101: e30236.
5.Allos BM (1997) J Association between Campylobacter infection and Guillain-Barré syndrome J Infect Dis Suppl 2: S125-128.
6.Kate O Poropatich, Christa L Fischer Walker, Robert E Black (2010) Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: a systematic review. J Health Popul Nutr 28: 545-552
7.Latov N (2022) Campylobacter jejuni Infection, Anti- GangliosideAntibodies, and Neuropathy. Journal of Microorganisms 10: 2139-2140.
8.Jasti AK, Selmi C, Sarmiento-Monroy JC, Vega DA, Anaya JM, et al. (2016) Guillain-Barré syndrome: causes, immunopathogenic mechanisms and treatment. Expert Rev Clin Immunol 12: 1175-1189.
9.Das S, Johnson DBJ (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. Immunother Cancer 7: 306-307.
10.Aoki S, Yasui M, Tajirika H, Terao H, Funahashi M, et al. (2020) Pembrolizumab-Induced Severe Neuropathy in a Patient with Metastatic Urothelial Carcinoma after Achieving Complete Response: Guillain-Barré Syndrome-Like Onset. Case Rep Oncol 13: 1490-1494.
11.Brzezinska BN, Higgins RV, Rungruang B (2021) Guillain- Barre Syndrome in a patient with uterine adenocarcinoma undergoing treatment with immune-checkpoint inhibitor therapy: A case report and review of the literature. Gynecol Oncol Rep 36: 100739.
12.Hashimoto R, Ueda T, Tsuji Y, Otsuka Y, Sekiguchi K, et al. (2020) Successful treatment of Guillain-Barré syndrome-like acute inflammatory demyelinating polyneuropathy caused by pembrolizumab with a combination of corticosteroid and immunoglobulins: a case report. Rinsho Shinkeigaku 60: 773-777.
13.Oguri T, Sasada S, Shimizu S, Shigematsu R, Tsuchiya Y, et al. (2021) A Case of Guillain-Barré Syndrome and Stevens- Johnson Syndrome/Toxic Epidermal Necrosis Overlap After Pembrolizumab Treatment. J Investig Med High Impact Case Rep.
14.Vikram Sangani, Mytri Pokal, Mamtha Balla, Ganesh Prasad Merugu, Sreedhar Adapa, et al. (2022) Pembrolizumab related Guillain barre syndrome, a rare presentation in a patient with a history of lupus and bladder cancer, J Community Hosp Intern Med Perspect 11: 388-392.
15.Han C, Ma JA, Zhang Y, Jiang Y, Hu C, et al. (2020) Guillain- Barre syndrome induced by pembrolizumab and sunitinib: A case report. Mol Clin Oncol 13: 38-42.
16.Ong S, Chapman J, Young G, Mansy T (2018) Guillain-Barré- like syndrome during pembrolizumab treatment, Muscle Nerve.
17.De Maleissye MF, Nicolas G, Saiag P (2016) Pembrolizumab- Induced Demyelinating PolyradiculoneuropathyN Engl. J Med 375: 296-297.
18.McNeill CJ, Fehmi J, Gladwin J, Christopher Price (2019) A rare case of Miller Fisher variant of Guillain-Barre Syndrome (GBS) induced by a checkpoint inhibitor. BMJ Case Rep 12: e229443.
19.Jacob A, Unnikrishnan DC, Mathew A, Thyagarajan B, Patel S (2016) A case of fatal Guillain–Barre syndrome from anti- PD1 monoclonal antibody use. J Cancer Res Clin Oncol 142: 1869-1870.
20.Sakoh T, Kanzaki M, Miyamoto A, Mochizuki S, Kakumoto T, et al. (2019) Ramsay-Hunt syndrome and subsequent sensory neuropathy as potential immune-related adverse events of nivolumab: a case report. BMC Cancer 19: 1220-1221.
21.Schneiderbauer R, Schneider Bauer M, Wick W, Enk AH, Haenssle HA, et al. (2017) PD-1 Antibody-induced Guillain- Barré Syndrome in a Patient with Metastatic Melanoma. Acta Derm Venereol 97: 395-396.
22.Pierrard J, Petit B, Lejeune S, Seront E (2019) Isolated adrenocorticotropic hormone (ACTH) deficiency and Guillain-Barré syndrome occurring in a patient treated with nivolumab. BMJ Case Rep 12: e230848.
23.Fukumoto Y, Kuwahara M, Kawai S, Nakahama K, Kusunoki S (2018) Acute demyelinating polyneuropathy induced by nivolumab. J Neurol Neurosurg Psychiatry 89: 435-437.
24.Kyriazoglou A, Liontos M, Papadopoulos C, Bilali A, Kostouros E, et al. (2019) Guillain-Barré Syndrome Related to Nivolumab: Case Report of a Patient With Urothelial Cancer and Review of the Literature. Clin Genitourin Cancer 17: e360-e364.
25.Mazzaschi G, Bordi P, Fioretzaki R, Gnetti L, Milanese G, et al. (2020) Nivolumab-Induced Guillain-Barré Syndrome Coupled With Remarkable Disease Response in a Case of Heavily Pretreated Lung Adenocarcinoma. Clin Lung Cancer 21: e65-e73.
26.Tanaka R, Maruyama H, Tomidokoro Y, Yanagiha K, Hirabayashi T, et al. (2016) Nivolumab-induced chronic inflammatory demyelinating polyradiculoneuropathy mimicking rapid-onset Guillain-Barré syndrome: a case report. Jpn J Clin Oncol 46: 875-876.
27.Nukui T, Nakayama Y, Yamamoto M, Taguchi Y, Dougu N, et al. (2018) Nivolumab-induced acute demyelinating polyradiculoneuropathy mimicking Guillain-Barré syndrome. J Neurol Sci 390: 115-116.
28.Gravbrot N, Scherer K, Sundararajan S (2019) Safe Transition to Pembrolizumab following Ipilimumab-Induced Guillain- Barré Syndrome: A Case Report and Review of the Literature. Case Rep Oncol Med.
29.Muralikrishnan S, Ronan LK, Coker S, Rauschkolb PK, Shirai K (2020) Treatment Considerations for Patients with Unresectable Metastatic Melanoma Who Develop Pembrolizumab-Induced Guillain-Barré Toxicity. A Case Report Case Rep Oncol 13: 43-48.
30.Touat M, Maisonobe T, Knauss S, Ben Hadj SO, Hervier B, et al. (2018) Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. J Neurology 91: e985-e994.
View PDF