Author(s): Opheelia Makoyo Komba*, Ulysse Minkobame, Pamphile Assoumou, Alain Boulende, Reteno Retno, Jacques Albert Bang and Jean François Meye
Context: The first assisted reproduction center (PMA) to emerge in Gabon was a private health facility in 2001. Then in 2016 a second private clinic followed suit. Despite this, in vitro fertilization (IVF) remained inaccessible to the greatest number because of the expensive cost of this care offer. Evidenced by the fertility rate in Gabon, which remains one of the lowest in the world, 4% in 2017. In order to provide a solution to this problem, the current government authorities have thought of setting up a fertility service in vitro in a public hospital.
Methodology: faced with the many difficulties observed, we proceeded as follows: Opinion of an expert in the equipment of an IVF center in November 2019; Architectural changes to the service; Staff training ; Order of missing equipment in October 2020; Establishment of collaboration and partnership; then organization of an IVF TEST session in November 2021 “free for patients”.
Result: The service laboratory is now fully functional. Staff have acquired skills and continue to improve. Over one year we carried out 44 cycles, we obtained 16 clinical pregnancies. That is a percentage of 36%. We noted 1 miscarriage, 3 deliveries, and 12 ongoing pregnancies including a twin.
Gabon is a country in Central Africa, located on the equatorial infertility belt [1]. Its population is around 2 million, with one of the lowest fertility rates in the world, 4% in 2017 [2]. Gabon’s fertility rate is declining every year, to the point that the country currently appears to be that of the Central African sub-region where women of childbearing age are the least fertile [3].
The first center to practice in vitro fertilization (IVF) in Gabon was a private structure in 2001. And since then this practice has remained the prerogative of private structures [4]. Reason why IVF remained inaccessible to the greatest number because of the expensive cost of this care offer.
In order to provide a solution to this problem, the current government authorities have thought of setting up an in vitro fertilization service within a public hospital.
Indeed, the mother-child university hospital center in question here was built in 2016, and was not opened to the public until 2 years later, in November 2018. It is a hospital of level 3 in which a medical procreation service was provided. Thus, when the hospital opened, this PMA service consisted of a minimum in terms of architecture and equipment.
Difficulties Noted When Opening
• The Architecture of the Service posed some problems for us. The service had not been built for this purpose and required some refitting. Indeed the arrangement of the different rooms was uncoordinated. As a result, traffic between the different areas did not meet the aseptic criteria.
• The Equipment delivered when the hospital opened was incomplete. The laboratory consisted of the following minimum: an inverted microscope for ICSI, a binocular magnifying glass, two incubators, two nitrogen tanks for Cryo preservation. This material had never been put into operation so its use was a discovery. Some consumables were expiring.
• The Staff (gynecologist and reproductive biologist) to work there was partly identified on the basis of their qualifications, their profit-sharing, voluntary work and a certain experience acquired during various external internships. Employees had to be trained on the job, this staff had to be identified in the various gynecology and laboratory departments within the hospital.
• Staff Training was done by building the capacities of the gynecologist and the biologist. The latter have followed diploma courses in infertility and reproductive biology in France. They benefited from immersion courses in fertility centers in Spain and Cameroon. The gynecologist and the biologist in turn composed and trained their collaborators identified in the hospital’s human resources. They were a doctor specializing in gynecology, a midwife, a secretary, and three laboratory technicians.
• Then Support by Equipment Providers for an IVF Center in November 2019. This is to help reorganize the architecture of the service and finally to comply with the standards of an IVF laboratory. The objective of this consultant was to draw up an inventory of the existing material and equipment. Provide the list and the type of device to order and the consumable to order (eg culture media, etc.). Thus the Order of the missing equipment was made a year later, in October 2020. It was a upright microscope; a centrifuge, an ESCO Miri incubator; a Storage tank for HIV-infected patients, a nitrogen container, and an oocyte suction pump.
• Then, the Establishment of a Collaboration and Partnership with a Gynecologist and a Foreign Embryologist for the Start of Activities. Their presence had several purposes. First, test the functionality of the service. Then their mission was to supervise, train, and evaluate intramurally the skills of the gynecologist and the Gabonese biologist. But also to train in better endoscopic surgical management of patients eligible for IVF who would need it beforehand.
• Finally, the Organization of an IVF TEST session in November 2021 “free for patients”. The objective is to verify the functionality of the IVF laboratory. For that:
• Five patients were selected. The main inclusion criteria were: women aged 21 to 34 having a body mass index (BMI) of 18 to 25 kg/ m2; diagnosis of infertility being a infertility original tubal or; infertile for ≥12 months. menstrual cycles _ regular 24 to 35 days, presumed ovulatory; On ultrasound transvaginal a uterus compatible with the function normal expected; documenting presence and accessibility _ adequate of both ovaries without sign of anomaly .
• By videoconference on ZOOM, the files are discussed and the doses to be administered agreed together. Meetings were scheduled on the days of the ultrasound and biological controls, ie D6; d 8; and d10
• The short multiple-dose antagonist protocol with estrogenprogestogen pill deprivation in order to plan the arrival of menstruation and the start of stimulation [5, 6]. The initial dose of gonadotropin was fixed at 150 IU for the first 5 days. Development follicular was monitored by ultrasound transvaginal after 5 days of treatment and then at least every 2 days. From the 6th day of stimulation, the dosage could be modified by 75 IU per adjustment [7]. The GnRH antagonist (cetrorelix: cetrotide*) was initiated on stimulation day 6 at a daily dose of 0.25 mg and continued throughout the gonadotropin treatment period. A single injection of 250 μg of hCG (choriogonadotropin alpha; Ovitrelle ; Merck Serono) was administered to induce final follicular maturation as soon as three ≥17 mm follicles have summer observed, i.e. the day on which the criterion is reached hCG or the next day . The goal of ovarian stimulation was to get eight to ten oocytes. The levy of oocytes occurred 36 ± 2 hours after administration of hCG.
• The first day of oocyte retrieval was scheduled the day after their arrival in Libreville. This is to allow the embryologist to adapt to the laboratory to test the microscope for ICSI and to prepare the culture media.
• The retrieved oocytes were assessed for cumulus mass appearance and stage of maturity. Metaphase II oocytes were inseminated using partner spermatozoa by ICSI 4 ± 1 hour after collection. All oocytes, embryos and blastocysts were evaluated daily by embryologists.
- Number of patients selected: 5
- Average age 27.2, average BMI 26.2, average CFA 19, average AMH 3.5.
- The five oocyte retrievals were carried out without difficulty, which validates the functionality of the operating room.
- Number of oocytes retrieved: 67; mean = 13 oocytes/patient
- Number of mature oocytes: 58/67
- Number of fertilized oocytes: 51
- Fertilization rate: 87.9%
- Number of embryos obtained in total: 50
- Number of vitrified embryos: 41
- Number of patients transferred: 3/5
- Freez all 2/5: 1 risk of hyperstimulation and 1 myoma obstructing the cavity.
- Number of embryos transferred = 7
- Stage of embryos transferred 5 D1 early cleaved and 2 D2
The percentage of having a positive pregnancy test at these embryonic stages is very low, we also wanted to test the transfers. The time allowed to our colleagues was very short. This justifies these transfers at early stages [8].
We organized the following sessions on a quarterly basis (Figure) which allowed us to formalize the start of this activity within the hospital.
After one year of activity:
- We performed 44 stimulation cycles
- We performed 48 embryo transfers and obtained 16 clinical pregnancies, a percentage of 33%.
- Among these 16 pregnancies we count:
- 6 pregnancies resulting from a frozen embryo transfer at the J5 blastocyst stage.
- 10 pregnancies resulting from a fresh embryo transfer at the J3 segmentation stage
- We noted one miscarriage, three patients gave birth and twelve pregnancies are ongoing.
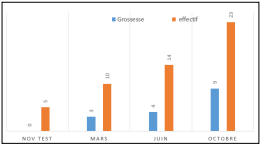
Figure: Results obtained at each session
The opening of the PMA service within the mother and child hospital in Libreville took 3 years. The determination of the main stakeholders was necessary for the success of this major project within a public hospital. Difficulties in terms of architecture and staff qualification were noted. Our experience is young, the skills are now acquired. The clinical pregnancy rate obtained after one year of practice is within the standards of 30%.
The authors declare no conflict of interest
