Author(s): Rania Hamed Shatla
Objective: Our study aims to estimate the incidence of non-convulsive status epilepticus (NCSE) in comatose children and to evaluate response to acute treatment with anticonvulsants.
Material and methods: This is a prospective observational cohort study conducted in Pediatric Intensive Care Unit (PICU) of Ain-Shams University. Eighty patients presented with Glasgow coma scale (GCS) <8 and/or the presence of abnormal movements or vital sign fluctuations were enrolled in the study. All were subjected to EEG monitoring for at least 1 hour within 24 hours of presentation. Patients diagnosed as NCSE received anticonvulsant treatment and were reevaluated clinically and by EEG after 1 week.
Results: Twenty four patients were diagnosed as NCSE (30%). EEG was normalized in 50% of patients after treatment.
Conclusion: NCSE is possible to be under-recognized in the PICU settings because of the pleomorphic clinical features. Clinical suspension of NCSE and EEG monitoring for critically ill comatose children will improve their outcome.
Non-convulsive seizures (NCS) and non-convulsive status epilepticus (NCSE) denote electroencephalographic (EEG) seizures activity without convulsive activity and often manifest as altered mental status or coma. Since there are no clinically evident convulsions, detection of NCS requires, by definition, EEG [1]. While there are no universally agreed upon diagnostic criteria, and no pathognomonic EEG changes in NCSE, persistent abnormalities without convulsive activity are the “gold standard” of diagnosis [2, 3].
Incidence figures of NCSE and the relative frequencies of its sub types vary across studies. This is probably due to the heterogeneous definitions and diagnostic criteria used, and the result of possible referral bias [4].
The impact of NCS and NCSE on neurologic outcome continues to be area of significant investigation, as increased seizure burden has been associated with higher mortality, longer pediatric intensive care unit (PICU) stays, and greater short term and long term disability in critically ill children [5].
Aim of the study: our study was designed to estimate the incidence of non-convulsive status epilepticus in comatose children and to evaluate their EEG and clinical response to acute treatment with anticonvulsants.
This is a prospective observational study that was conducted in Pediatric Intensive Care Unit (PICU) of Ain Shams University, Cairo, during the period from February, 2013, till May, 2014. Eighty patients were enrolled, their age ranged from 2 months -18 years .All were presented with Glasgow coma scale <8 that had an unclear etiology or was disproportionate to a known medical condition, and/or the presence of abnormal movements or vital sign fluctuations [1 & 6]. Neonates and patients with underlying metabolic disorder were excluded from the study.
For all included patients’ detailed history taking including onset and duration of coma, presence of preceding symptom as; isolated seizure , convulsive status epilepticus (CSE). We search for specific etiology as epilepsy, (current anti-epileptic medication used and whether patient abruptly discontinued medication or was uncontrolled), hypoxic-ischemic encephalopathy or traumatic brain injury or stroke or CNS infection or neurosurgery or other structural brain disease. As well as any major illness (preexisting medical and neurological conditions) or previous chronic disease were also recorded to explore probable underlying cause of the condition.
To diagnose NCSE there has to be, at the very least, EEG evidence of seizures. So, EEG monitoring for at least one hour was done, at time of presentation using Nicolet biomedical, a twenty-one gold- over-silver scalp surface electrodes were positioned according to the international 10-20 system and affixed with gel.
NCSE was defined as a state of impaired consciousness with either a single 30-minute electroencephalographic seizure or a series of recurrent independent electroencephalographic seizures totaling more than 30 minutes in any 1-hour period (50% seizure burden) [1]. Using this definition and with the availability of EEG monitoring in our neurological intensive care unit, we were able to diagnose 24 NCSE patients over a period of 15 months
All our patients subjected to through clinical examination to detect any abnormal neurologic examination as presence of ocular movement abnormalities including nystagmus, hippos, and sustained eye deviation in any direction. We also observed any subtle motor activity not only persistent muscle twitches in the extremities or face but also automatisms.
NCSE was categorized into focal and generalized epileptic activity based on the EEG monitoring, the epileptiform activity or focal EEG changes appeared to be frontal, temporal, bilateral synchronous discharge or focal to bilateral and then generalized pattern [7, 8].
Patients with NCSE were reevaluated clinically by Glasgow coma scale and EEG after 1 week of treatment protocol of CSE according to National institute for Health and Care excellence guidelines, 2013.
Statistical analysis was done using manual methods to calculate percentage, mean and standard deviation of the obtained data of the patients.
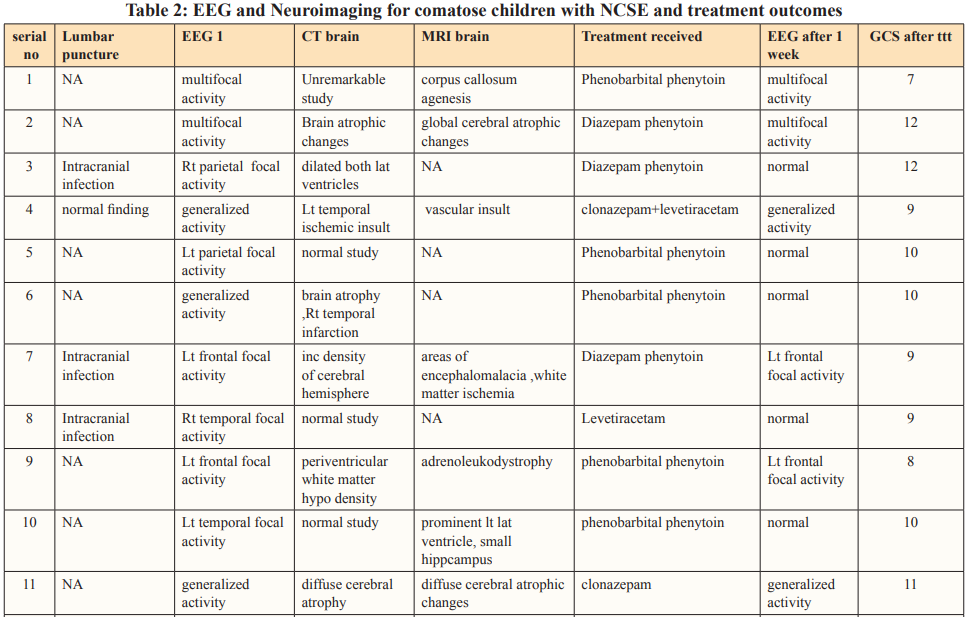
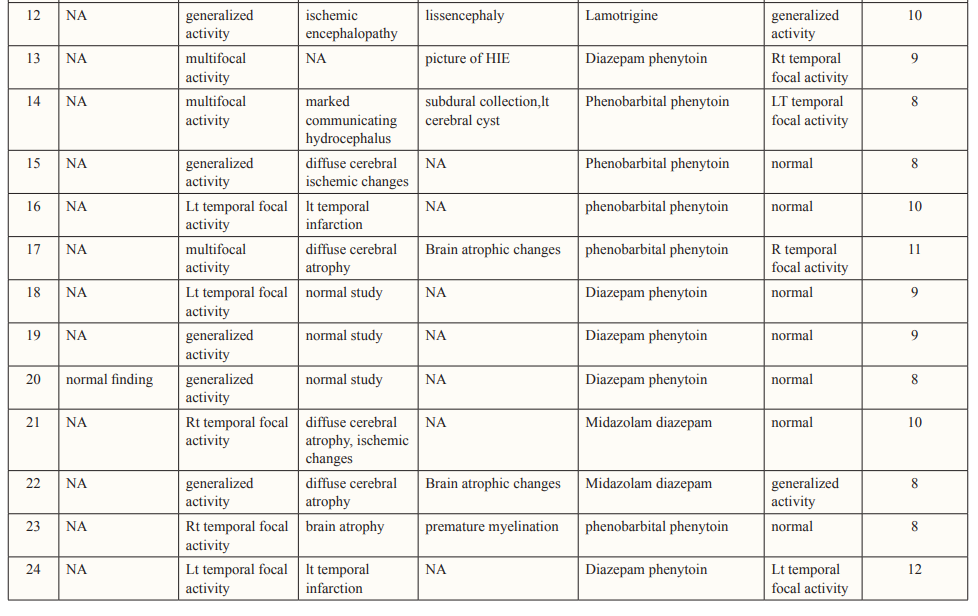
Clinical manifestations, laboratory results, and cranial neuroimaging findings of the included patients are summarized in Tables 1 and 2.
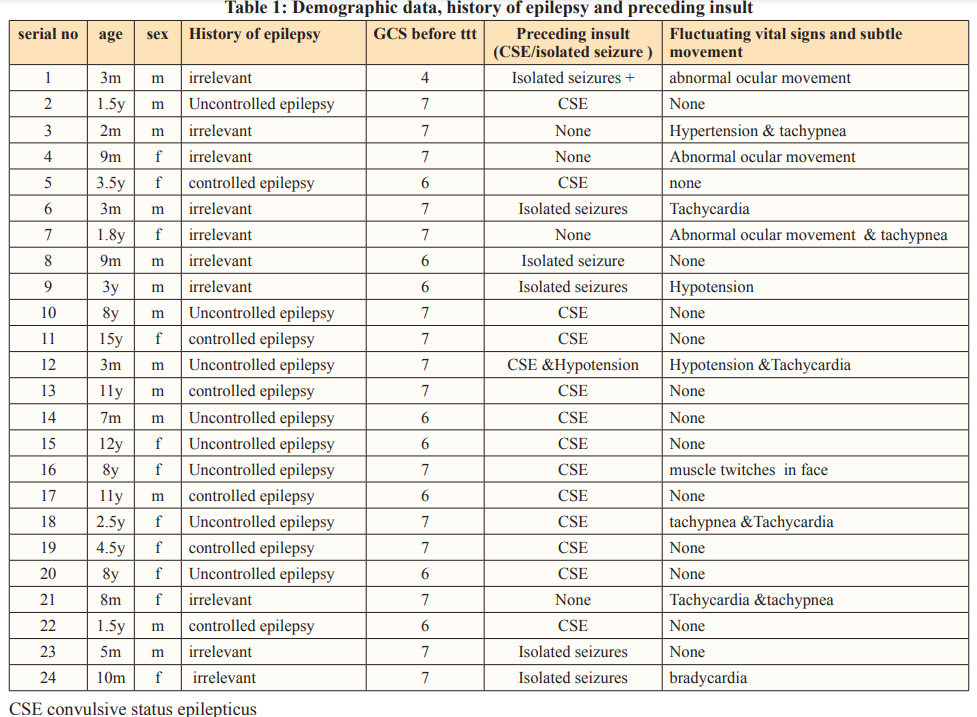
Out of eighty patients enrolled in the study with disturbed conscious level (coma), we were able to diagnose twenty four patients (30%) as NCSE by fulfilling EEG and clinical criteria. They were 13 male and 11 female with mean age 2.70± 3.24 years. Whereas ten patients were less than one year old, seven patients were between one and five years, and seven patients older than five years old.
Regarding patients with NCSE; eleven were known epileptic patients. Four of them were diagnosed as primary epilepsy and seven of them were symptomatic/ cryptogenic. However, all of them experienced CSE prior to NCSE. Notably, eight of them had history of uncontrolled epilepsy.
Another six had isolated seizure episode before developing NCSE. None of them was known epileptic. Reviewing their history showed global delay with abnormal neuroimaging except for one patient who was completely normal and was diagnosed with intracranial infection.
Four patients in our study developed NCSE without prior CSE or isolated seizure. Of these two had intracranial infection, and they were presented with coma and unexplained fluctuating vital signs and/or subtle convulsions, the other two patients developed NCSE de novo. Notably both were globally delayed and had and underlying ischemic brain insult.
In patients with NCSE; subtle movements were observed in five patients, and unexplained vital sign fluctuations in eight patients. Prior convulsions occurred in five (out of eight) patients’ with vital sign fluctuations and only one patient (out of three) with subtle movement had no preceding convulsions.
EEG done within 24 hours of the last seizure revealed focal activity in 11 patients, multifocal activity in five patients and generalized activity in eight patients.
Based on the results of neuroimaging NCSE four epileptic patients had normal imaging and ten epileptic patients had abnormal neuroimaging. Also, NCSE occurred in nine non epileptic patients all had previously underlying brain insult. Post treatment evaluation showed a significant improvement in GCS 9.3±1.6 as well as EEG.
The overall population incidence of NCSE was estimated at 5.6-18.3 per 100,000 individuals per year on the basis of five major epidemiological studies [8]. It’s worth noting that, large prospective studies monitoring all children diagnosed with brain insults who are admitted to an intensive care unit (ICU) are lacking. Shahwan et al study showed a wide range of variability of NCSE (7%-47%) in critically ill children in pediatric intensive care units or emergency departments undergoing cEEG monitoring [6].
In our study twenty four patient (30%) out of 80 patients were diagnosed as NCSE which is nearly similar to 35%, 33.9%, and 33% reported by other studies [9-11]. On the other hand two different studies revealed that NCSE was the underlying cause of coma in 16.3% (out of 141) and 8% of more than 200 comatose patients, respectively [12,13]. Studies have shown that younger age is associated with a higher incidence of NCS and NCSE. Patients less than 18 years of age may be at higher risk than adults for NCSE. Furthermore, within the pediatrics age group, neonates and infants represented a particularly higher risk [15]. Our work echoes similar finding, as mean age of patients with NCSE was 2.70±3.24 years. In accordance to our result that 41.6% of patients with NCSE were less than one year. Other studies, observed NCSE in less than one year of age in 30%, 39% and 36%, respectively [12-14]. On the other hand, Greiner et al reported NCSE in patients with mean age 7.8 years and 7.9 years, respectively [16].
In our study, male: female ratio among NCSE was 1.2:1 which is nearly similar to 1.3:1 observed and not in accordance with increased incidence in males reported in other studies, (1.7-1.9:1), respectively [10-12].
The Glasgow coma scale of our patients ranged 4-7 with mean value 6.54±1.69 which was similar to a study done in which the GCS was below eight, however, observed that 19 patients with NCSE had GCS above nine and 11 patients below nine, so may be in further studies patients with higher GSC should be investigated for NCSE [1 & 17].
Several studies reported that one of the major clues to the diagnosis of NCSE is presence of subtle clinical signs such as eye fluttering and various automatisms (involuntary, autonomic movements that occur with an alteration in consciousness, including those that are gestural (for example, picking movement with the fingers) or oro- alimentary, for example, lip smacking or stuttering)[3,13,18,19] . In our study, five patients with NCSE had abnormal movements (three patients with abnormal ocular movements and two patients with muscle twitches in face and extremities). Eight patients (33.33%) had fluctuations in vital signs (blood pressure, heart rate and respiratory rate), compared to study done by Abend et al in which abnormal movements and vital sign fluctuations were observed in 8 and 3 (out of 100 patients), respectively [1].
NCSE can occur de novo or develop after a convulsive event and should be especially considered in patients who fail to awaken after generalized seizures [3]. In our study, convulsive status epilepticus and isolated seizures occurred in 58.3% and 20.8%, respectively. Other studies documented that 12% to 53% of CSE converts to electrographic status epilepticus without clinical signs [20-23]. On the other hand; prior studies have also reported the occurrence of NCS and NCSE with or without preceding convulsions or CSE [15, 24].
The etiology of NCSE is poorly understood and apparently very heterogeneous. In many cases, NCSE appears to correspond to a conversion of an existing epilepsy syndrome presenting as self- limiting seizures. It is probable that poor initial control of seizures favours conversion to NCSE, as is well documented for CSE [25].
In our study, history of epilepsy was observed in 14 patients, 11 of them were uncontrolled, this is in agreement with Shahwan et al [6] who reported that 6 of 7 with seizures detected had prior epilepsy diagnoses and witnessed convulsions. Moreover, Walker et al [8] noted that between 30% and 50% of subjects with NCSE had a prior history of epilepsy. On the other hand, other studies have reported that between 47% and 65% of children with NCSE do not have preexisting epilepsy compared to 41.6% observed in our study [10, 15, 16].
Also we found that fifteen patients (62.5%) with NCSE had abnormal development which is nearly similar to 67% noted in the study by Greiner et al [16].
The role of EEG recording duration in NCSE was a great debate, yet, many studies recommended the use of abbreviated EEGs in the emergency room and of serial EEGs or continuous EEG (cEEG) monitoring in intensive care units (ICUs) has increased the number of diagnosed comatose NCSE [24-28].
Non convulsive seizures are common during cEEG in critically ill children (seen in 44% of patients) [24]. Half are detected in the first hour of recording [1,23], whereas 20% are not detected until after more than 24 hours of recording [23], showing that many children with NCS or NCSE would not be identified without prolonged cEEG recording [1]. In our study EEG was done for at least on hour within first 24hrs of initial presentation for all patients, showing focal activity in 11 patients (45.8%), generalized activity in eight patients (33.3%) and multifocal activity in five patients (20.8%). However, Greiner et al [16], studied 26 patients with NCSE and ictal abnormalities included generalized (n = 15), focal (n = 9), and multifocal (n = 2) distributions. Also, another study revealed generalized ictal discharges in 59 episodes (69%), diffuse with focal predominance in 15 (18%) and focal in 11 (13%) [7].
MRI or computerized tomography should be offered to all patients at first presentation of NCSE [29]. In our study CT brain was done for 22 patients and MRI was done for 12 patients with NCSE showing normal study in five patients, old ischemic insult in eight patients, brain atrophy in six patients and hydrocephalus in two patients, compared to the results of neuroimaging done in Tay et al [10]. Which were normal study in three patients out of 19, brain atrophy in three patients, hydrocephalus with shunt in place in one patient.
Randomized controlled trials of treatments for NCSE are lacking so treatment of NCSE is directed by the cause. Most conventional antiepileptic drugs (AEDs) have been reported to be effective for some patients. Refractory NCSE has been reported to respond to midazolam, propofol or pentobabrbitol [30]. Treatment of NCSE in comatose patients in our study followed the scheme of commonly used in CSE, with intravenous benzodiazepines as first choices, followed by phenytoin, phenobarbital or valproate. Yet, many other lines of treatment were not used due to lack of availability of these drugs in our country. After treatment, the GCS showed improvement 9.3±1.6 compared to initial one with 50% normalization of EEG. In a recent multicenter study from 11 sites in North America retrospectively reviewed a total of 550 consecutive children in pediatric intensive care units who underwent EEG monitoring. They concluded that, electrographic seizures are common among children in the pediatric intensive care unit, particularly those with specific risk factors. Electrographic status epilepticus occurs in more than one-third of children with electrographic seizures and is associated with higher in-hospital mortality [31].
NCSE is under-recognized at least (30%) in the PICU settings because of the pleomorphic clinical features. It must be suspected in any patient with disturbed level of consciousness, subtle movements and abnormal vital signs fluctuation to be confirmed by cEEG. Early treatment of NCSE may improve the outcome of comatose children at PICU. Multicenter studies are warranted to establish guidelines for management of NCSE in comatose children at PICU.
1.Abend NS, Gutierrez-Colina AM, Topjian AA (2011) Nonconvulsive seizures are common in critically ill children. Neurology 76: 1071–7.
2.Meierkord H, Holtkamp M (2007) Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 6: 329-39.
3.Chang AK, Shinnar S (2011) Nonconvulsive Status Epilepticus. Emergency Medicine Clinics of North America 29: 65-72.
4.Litt B, Wityk RJ, Hertz SH, Mullen PD, Weiss H, et al. (1998) Non-convulsive status epilepticus in the critically ill elderly. Epilepsia. 39: 1194-202.
5.Wilson CA (2015) Continuous electroencephalogram detection of non-convulsive seizures in the pediatric intensive care unit: review of the utility and impact on management and outcomes. Transl Pediatr 4: 283-9.
6.Shahwan A, Bailey C, Shekerdemian L, Harvey AS (2010) The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia 51: 1198–204.
7.Granner MA, Lee SI (1994) Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia. 35: 42-7.
8.Walker M1, Cross H, Smith S, Young C, Aicardi J, et al. (2005) Nonconvulsive status epilepticus: Epilepsy Research Foundation workshop reports. Epileptic Disord 7: 253-96.
9.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, et al. (2012) Nonconvulsive Status Epilepticus: The Encephalopathic Pediatric Patient. Pediatrics 129: 748–55.
10.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI (2006) Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 47: 1504-9.
11.Hosain SA, Solomon GE, Kobylarz EJ (2005) Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol 32: 162–165.
12.Saengpattrachai M, Sharma R, Hunjan A, Shroff M, Ochi A, et al. (2006) Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia 47: 1510-18.
13.Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, et al. (2000) Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 54: 340–5.
14.Abend NS, Dlugos DJ (2007) Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol 37: 165–70.
15.Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA (2001) Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology 57: 1036-42.
16.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, et al. (2012) Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics 129: 748–55.
17.Kuchta J, Klug N, Ernestus RI (2009) Nonconvulsive status epilepticus as a possible cause of coma in neurosurgical intensive care. Cent Eur Neurosurg 70: 176-9.
18.Kaplan PW (1996) Nonconvulsive Status Epilepticus in the Emergency Room Epilepsia 37: 643-50.
19.Kaplan PW, Stagg R (2011) Frontal lobe nonconvulsive status epilepticus: a case of epileptic stuttering, aphemia, and aphasia--not a sign of psychogenic nonepileptic seizures. Epilepsy Behav 21: 191-5.
20.Jaitly R, Sgro JA, Towne AR, Ko D, DeLorenzo RJ (1997) Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol. 14: 326-34.
21.Treiman DM (1993) Generalized convulsive status epilepticus in the adult. Epilepsia.34: S2-11.
22.DeLorenzo RJ, Waterhouse EJ, Towne AR, Boggs JG, Ko D, et al. (1998) Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 39: 833-40.
23.Knake S1, Rosenow F, Vescovi M, Oertel WH, Mueller HH, et al. (2001) Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 42: 714-8.
24.Jette N, Claassen J, Emerson RG, Hirsch LJ (2006) Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 63: 1750-5.
25.McCoy B, Sharma R, Ochi A, Go C, Otsubo H, et al. (2011) Predictors of nonconvulsive seizures among critically ill children. Epilepsia 52: 1973-8.
26.Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR (2004) Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol 61: 1090-4.
27.Narayanan JT, Murthy JM (2007) Nonconvulsive status epilepticus in a neurological intensive care unit: profile in a developing country. Epilepsia 48: 900-6.
View PDF