Author(s): Kehinde Alare
Aims: the aim of the research is to look to the neurological and molecular mechanism of diabetic neuropathy and also suggesting a better way of management.
Methods: Critical reviews of the relationship between hyperglycemia in diabetes with polyol pathway, hexose monophosphate shunt and de novo synthesis of sphingolipids and how this relationship could be involved in diabetic neuropathy.
Results: The research gave rise to some factor causing diabetic neuropathy such as osmotic stress, oxidative stress , dymelination and are gotten from the method above.
Conclusion: This research pointed out decrease NADPH available for de novo synthesis of sphingomyelin as result of increase consumption by polyol pathway and decrease production by hexose monophosphate shunt as the main factor responsible for diabetic neuropathy
Pathology of the neurons may be due to different alteration in the structural, electrical and chemical components of the nerve cells.
In some cases, neuropathy has been associated with the myelination process of the neurons in which myelin sheath the protective covering of the neurons are produced leading to reduction in protection and electrical conductivity of the neurons.
Peripheral neuropathy is the most common and intractable complication of diabetic [1].
In accordance to research made by Pirat, prevalence of diabetic neuropathy ranges from 7% within 1year of diagnosis to 50% for those with diabetes for greater than 25years [2].
Complications of these neuropathy if not taken care of could lead to amputation or even reduce the life expectancy in patients with cardiac autonomic nervous system affected.
The aims of this research articles include the following: To critically review the cause of neuropathy experienced in diabetic patient so the adequate knowledge of it pathogenesis will gives the adequate measures to the management in order to completely alleviate the symptoms and to restore normal neurophysiology in patients.
To look into the neurological and molecular causes of diabetic neuropathy in order to pin point some causes that researchers might have not been considering which’s been crucial in the development of neuropathy particularly in diabetic patients.
To highlight the reason why pyridoxine that’s been helpful in the management of neuropathy in non diabetic patients has not been helpful in diabetic patients.
Critical review of the relationship between the hyperglycemia experienced in diabetes melitus and it’s interference with body’s metabolism particularly those relating with normal physiological functioning of neurons.
From our thoughts we pointed out deaths and gross demyelination of the neurons to be the cause of neuropathy so we critically sorted out molecular mechanisms that could result in death or gross demyelination of neurons in diabetic patients.
We looks into the effects of hyperglycemia has on some metabolic pathways in the body like polyol pathway, hexose monophosphate shunt and de novo synthesis of sphingolipids and we arise at the discussion below
Our findings is discussed as follows:
The symptoms of neuropathy seen in diabetic patients occurred as a result of defects in the electrical conductivity and structure of the neurons and even death of the nerve cells.
In normal physiology, nerve conduction is done in a way that impulses are transmitted from the dendrites through the axon to the dendrites of another neuron. There myelin sheaths covering the outer part of the axon and are separated by nodes of Ranvier . These myelin sheaths are electrical insulators which obstruct the flow of impulses across the axon ; these transmitted impulses have to jump over the myelin sheaths thereby increasing the velocity of conduction. Also, these myelin sheaths serve as protection to the neurons from external forces that can kill or alter the normal physiology of the neurons.
Myelin sheaths are produced through a process of myelination done by Schwann cells in the peripheral nervous system and by oligodendrocytes in the central nervous system. Any pathology in the production of these myelin sheaths in one of the major causes of neuropathy which is prominent in some disease conditions known as “demyelination diseases” and this also occurs in diabetic neuropathy and the mechanism is explained under the molecular basis section.
Demyelination of nerve cells is a normal phenomenon that occurs in almost all neurons but it is being compensated for by remyelination process that occurs almost concurrently in which new myelin sheaths are produced by Schwann cells and oligodendrocytes as seen in research made on cats and if the process of remyelination is being hindered by any factor, it will result in gross demyelination if the neurons as seen in demyelination disease (e.g multiple sclerosis) and also in diabetic neuropathy [3].
Effects of Demyelination
As a result of the destruction of myelin sheaths that occurs in demyelination, there is an effect on the conduction of nerve impulses which more prominent in the peripheral nervous system [4]. There is reduction in the conducting velocities of nerve impulses in affected neurons which result in reduction in sensation or activities of the neurons involved.
Myelin sheath which is the protective coating of the neurons and it’s damage exposes the nerve cells to harsh environmental conditions which can lead to death of some the nerve cells before adaptation will occur in others.
The gross effect of the demyelination in diabetic neuropathy is
evidence as :
Peripheral neuropathy symptoms include :
• Numbness or reduced ability to feel pain or temperature
changes.
• Tingling or burning sensation
• Sharp pains or cramps
• Increased sensitivity to touch for some people, even a
bedsheet’s weight can be painful.
• Serious foot problems such as ulcers , infections, bone and
joint pains.
Autonomic Neuropathy Symptoms Include:
• Hyperglycemia unawareness
• Bladder or bowel problems
• Gastroparesis
• Reduction in libido
Diabetic Polyradiculopathy • Severe pain in thigh and gluteal region. • Difficulty rising from a sitting position • Stomach pain.
Monofocal Neuropathy
Diabetic neuropathy has been molecularly linked with excessive glucose in circulation and here are some explanation to the mechanism of diabetic neuropathy
This studies is presenting reduction in the available reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) as a result of hyperglycemia as the major cause of diabetic neuropathy as well as osmotic and oxidative stress leading neuroedema and neuronal death respectively
In uncontrolled hyperglycemia, glucose in the presence of aldose reductase (AR) enters polyol pathway consuming the available NADPH to give sorbitol and the presence of sorbitol dehydrogenase, sorbitol is being converted to fructose.
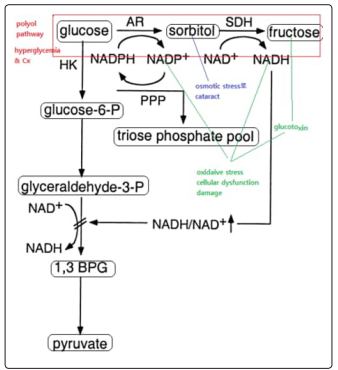
Figure 1: Polyol pathway linked with glycolysis
Sorbitol produced causes osmotic stress to the neurone leading oedema while fructose, NADH and NADP+ produced in polyol pathway causes oxidative stress to the neuron.neuropathic changes even in aldose reductase (AR) deficient diabetic mice [1].
Osmotic and oxidative stress caused by polyol pathway can be causing neuropathy but couldn’t account for demyelination of neuron though they may account for neuronal death. The consumption of NADPH in polyol pathway is the major concern to this research. One could say that the NADPH consumed in polyol pathway will be compensated for by hexose monophosphate shunt( the major pathway of NADPH generation in the body). But, in the presence of excessive amount of glucose (as in hyperglycemia),glucose-6-phosphate dehydrogenase (G6PD) which is the major enzyme in the hexose monophosphate shunt is inhibited by glucose when in excess [5].
Figure 2: hexose monophosphate shunt
The inhibition of hexose monophosphate shunt by excess glucose will not only affect production of NADPH but also hinder the production of reduces glutathione which thereby predisposes he neurons to oxidation by free radicals generated by glycolysis or polyol pathway causing oxidative stress. Inhibition of hexose monophosphate shunt also exposes the neuron to nitrosative stress caused by nitric oxide (NO) generated by the neurons from NOsynthase [6]. This not aldose reductase dependent it occurs both in the presence and absence of aldose reductase
As mentioned earlier, increase in consumption of NADPH in polyol pathway and decrease in its production due to inhibition of hexose monophosphate shunt is the main factor accounting diabetic neuropathy as it’s responsible for demyelination of the neuron.
The de Novo synthesis of the sphingomyelin which is a major component of myelin sheath is greatly NADPH dependent as NADPH is required in the formation sphinganine .
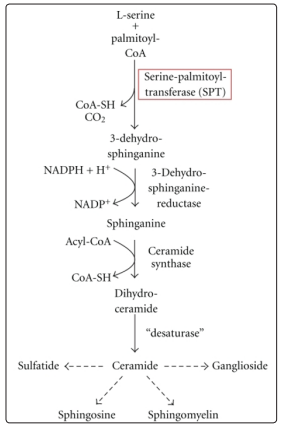
Figure 3: De Novo synthesis of sphingolipids Adapted from “Intracellular APP Domain Regulates Serine-Palmitoyl Transferase Expression and Is Affected in Alzheimer’s Disease” M Grimm, S Grösgen ,2011, International Journal of Alzheimer’s Disease pg3 Copyright by SAGE-Hindawi Access to Research 2011. Reprinted with permission.
There is increase in consumption of NADPH by polyol pathway and reduction in production of NADPH by hexose monophosphate shunt inhibition leading to reduction in available NADPH required for de novo synthesis of sphingomyelin which affect production of myelin sheath. Some physician do think that neuropathy in diabetic patients may be manage with pyridoxine as in normal patients in which lack of pyridoxal phosphate which is a major co-enzyme in the de novo synthesis of sphingomyelin is the major cause of neuropathy in them.
But this has not been very helpful patient because lack of NADPH is the major factor responsible for diabetic neuropathy [7].
The cumulative effects of osmotic stress caused by sorbitol produced in polyol pathway oxidative stress due to increase free radicals produced in polyol and glycolytic pathway and reduction in the production of reduces glutathione by hexose monophosphate shunt , nitrosative stress due to nitric oxide synthesis and inhibtion of hexose monophosphate shunt and demyelination due to reduction in available NADPH requires the de novo synthesis of sphingomyelin all result in the symptoms of diabetic neuropathy.
According to this research, the suggested manao of neuropathy
in diabetivmc patients include :
• Aldose Reductase Inhibitors: Aldose Reductase inhibitors
blocks the action of aldose reductase in conversation of
glucose to sorbitol thereby inhibiting the entire polyol
pathway and will be helpful in the management of diabetic
neuropathy although research have shown that AR inhibitors
are limited in the management of diabetic neuropathy [1]. This
limitation has been explained earlier, polyol pathway is not
the only pathway involved in diabetic neuropathy but the
usage of AR inhibitors will be helpful in preventing osmotic
stress and reducing oxidative stress and demyelination.
• NADPH Generating Supplements (e.g Citrate): The usage
of supplements like citrate which will generate NADPH by
other means will help increasing the availability of NADPH.
Citrate enters citric acid cycle (CAC) to generate NADPH
thereby increasing the bioavailability of NADPH for de novo
synthesis of sphingomyelin , thereby inmproving myelination
process in both central and peripheral nervous systems.
Including this in the management of diabetic neuropathy
will be very helpful.
• Controlled Hyperglycemia: To us, this is the gold standard
in the management of diabetic neuropathy because polyol
pathway will be properly controlled and inhibition of hexose
monophosphate shunt will be minimal. This caters for all
the four causes of diabetic neuropathy mentioned in this
research, osmotic stress, oxidative stress, nitrosative stress and
demyelination. However, in patients whose hyperglycemia
couldn’t be controlled; the combined therapy of AR inhibitors
and NADPH supplements(citrate) should be helpful in the
management of diabetic neuropathy in them.
The neurological mechanism of diabetic neuropathy which is neuronal death or demyelination of nerve cells has been linked molecularly to osmotic and oxidative stress being caused by polyol pathway and demyelination as a result of decrease in the bioavailability of NADPH due to inhibition of hexose monophosphate shunt.
This research has proven that decrease in the amount of available NADPH has the major factor responsible for demyelination and neuropathy in diabetes and has also suggested the modalities of management of diabetic neuropathy.
In conclusion, diabetic neuropathy could be avoided by proper monitoring of blood glucose and by taking NADPH supplements in patients with higher level of glucose in the blood [8].
